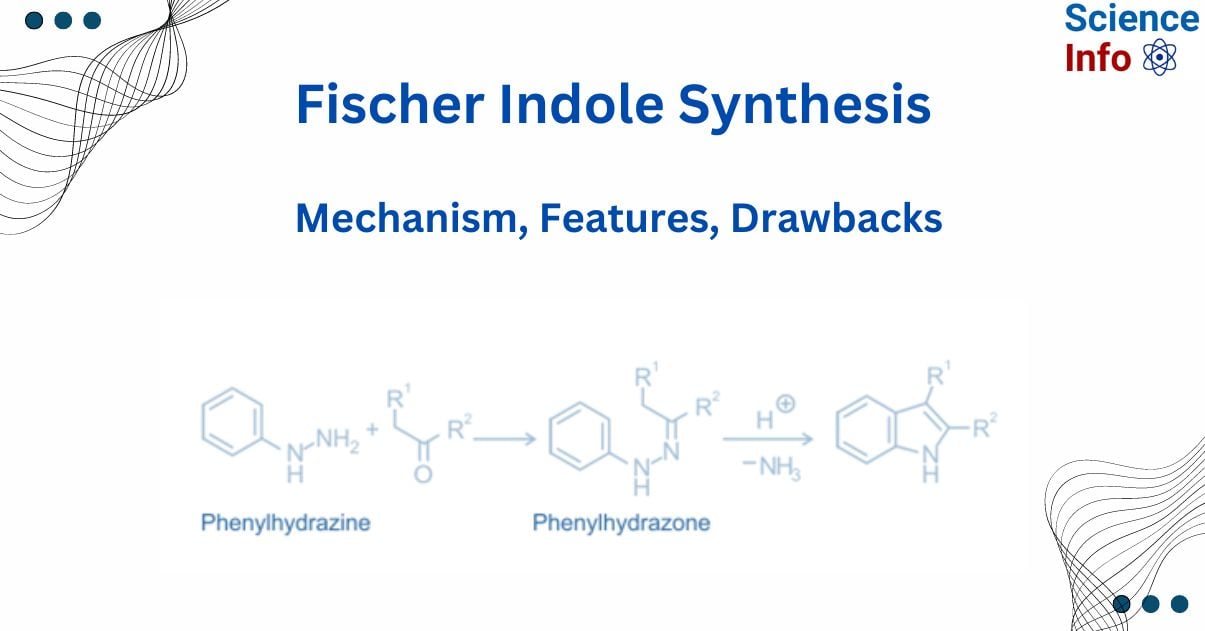
The Fischer indole synthesis is one of the oldest synthetic processes, and because of its versatility, it is commonly used to prepare substituted indoles. It was invented by Emil Fischer in 1883. It is a well-known technique for synthesizing substituted indoles from aryl hydrazines, as well as substituted aldehydes or ketones under acidic conditions. The acid catalyst is usually a Lewis acid, such as zinc chloride or aluminum chloride.
Indole is a benzo[b]pyrrole produced by combining the benzene ring with the 2,3 positions of the pyrrole nucleus. The term “Indole” comes from the word “India,” as the heterocycle was initially isolated from a blue dye called “Indigo” made in India around the 16th century. In 1886, Adolf Baeyer isolated Indole by pyrolyzing oxindole with Zn dust. Oxindole was produced by reducing isatin, which was then isolated by oxidizing Indigo. Commercially, indole is made from coal tar.
The indole ring system is a key scaffold in a wide range of biologically significant compounds, hence there are several approaches to accessing this heterocyclic ring. The indole ring system is widely distributed in biological systems and is regarded as a favored heterocycle in medicinal chemistry.
These are a significant class of heterocyclic compounds with various biological and pharmacological uses, including the treatment of cancer, depression, emesis, hypertension, inflammation, and migraines. As a result, it has been extensively exploited in the production of pharmaceuticals and other bioactive compounds.
Indole is both a weak base and a weak acid. It polymerizes with strong acids and interacts with potassium hydroxide and Grignard reagents.
Interesting Science Videos
Fischer indole synthesis
Emil Fischer developed this reaction in 1983, and it has since become the most used way of preparing indoles. It is the reaction of an aryl hydrazine with an aldehyde or ketone in the presence of an acid catalyst. This acid catalyst may be either a Lewis acid or a Bronsted acid. Lewis acids include zinc chloride, iron chloride, and boron trifluoride. Bronsted acids include polyphosphoric acid, hydrochloric acid, sulfuric acid, and p-toluenesulfonic acid.
The indole nucleus is formed by cyclizing an arylhydrazone, which is produced from aryl hydrazine and aldehyde or a ketone using an acid catalyst or thermally.
The breadth of functional groups that are tolerated, the easy accessibility of the starting materials, and the absence of a need to isolate the hydrazone intermediate are among the many benefits of the Fischer indole synthesis.
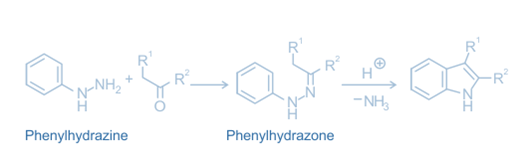
Mechanism of Fischer Indole synthesis
The arylhydrazone formed by the condensation of an aryl hydrazine and a carbonyl molecule is protonated and isomerized, yielding the enamine tautomer. The protonated enamine tautomer then experiences an irreversible electrocyclic rearrangement known as [3,3]-sigmatropic rearrangement, which breaks the N-N bond.
The resultant imine is formed from a cyclic amino acetal or aminal, which then eliminates ammonia under acidic catalytic conditions to yield an energetically advantageous aromatic indole.
Hydrazine reacts like an amine with a carbonyl molecule to produce hydrazone. The cyclic intermediate is subsequently rearranged, losing a proton from nitrogen and forming a new C=C bond, resulting in the creation of the substituted indole product.
Special Features of Fischer Indole Synthesis
- This synthesis can alternatively be carried out by immediately allowing an equimolar combination of aryl hydrazine and the aldehyde or ketone to indolization conditions without first separating the hydrazone.
- Aryl hydrazones can also be formed by reducing the corresponding aryldiazonium salt or N-nitro arylalkylamine, or through a palladium-mediated coupling process in the presence of a carbonyl moiety. These procedures are used when the aryl hydrazones are unstable or poisonous.
- Unsymmetric ketones produce two region-isomeric 2,3-displaced indoles with region-specific selectivity determined by medium acidity, hydrazine substitution, and steric effects.
- 1,2-diketones can produce both mono-indoles and bis-indoles, which are often produced by strong acid catalysts in refluxed alcohols.
Drawbacks of Fischer Indole Synthesis
- Fischer Indole Synthesis reaction is extremely sensitive to reaction parameters such as temperature, acid strength, and reaction time. This can influence the yield and purity of the final product.
- Certain functional groups, such as extremely reactive and acidic functional groups, are not appropriate for this reaction and may result in unwanted side reactions or the breakdown of the intended product.
- Fischer Indole Synthesis reaction fails with acetaldehyde and hence cannot be utilized to synthesize indole itself. It is possible to substitute keto acid with pyruvic acid and decarboxylate the result to produce indole.
- The reaction can produce undesirable byproducts like aldol condensation products or Friedel Crafts products, lowering the yield of the desired indole product.
References
- https://www.bhu.ac.in/Content/Syllabus/Syllabus_300620200422112348.pdf
- https://byjus.com/chemistry/fischer-indole-synthesis/
- https://www.sciencedirect.com/science/article/abs/pii/S0065272522000563
- https://www.sciencedirect.com/science/article/abs/pii/S0065272522000563?via%3Dihub
- https://www.organic-chemistry.org/synthesis/heterocycles/benzo-fused/indoles.shtm
- https://www.britannica.com/science/indole
- https://www.chemistrylearner.com/fischer-indole-synthesis.html
