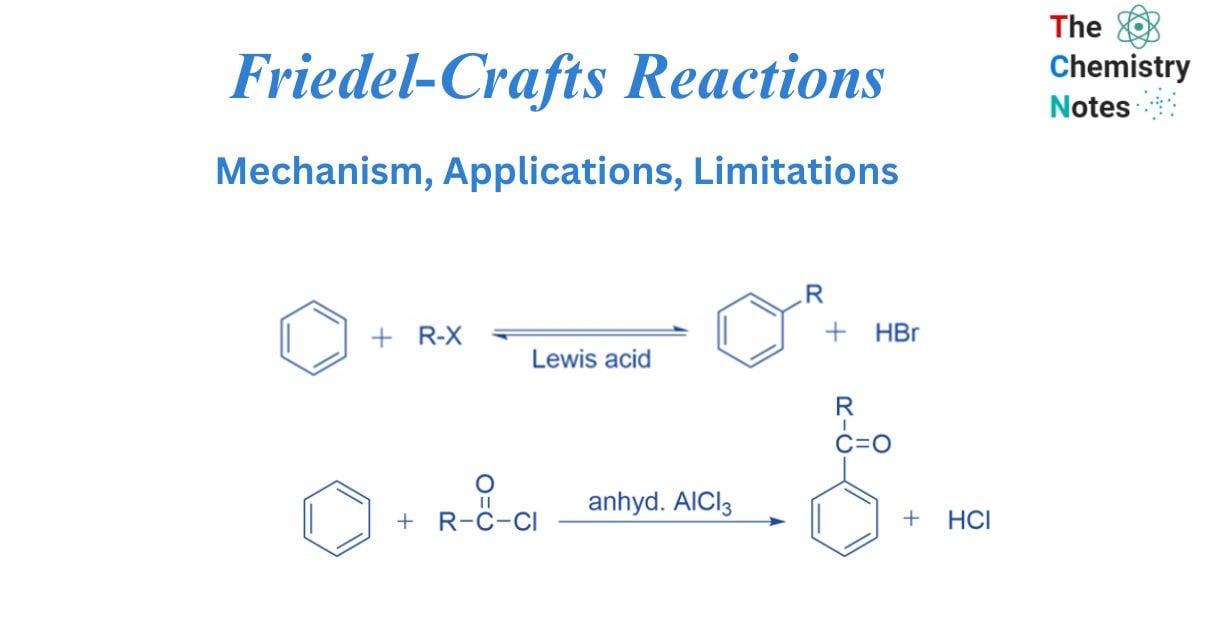
Friedel-Crafts reactions generate new C-C bonds in organic molecules. The aromatic molecule is alkylated or acylated in the presence of an acid catalyst, such as AlCl3, BF3, ZnCl2, FeCl3, and so on. In Friedel-Crafts reactions catalyst’s role is to produce the attacking particle, an alkyl or an acyl cation.
An acylation reaction is one form of Friedel-Crafts reaction in which an RC=O group – an acyl group – is replaced for a hydrogen atom (bonded to a carbon atom) in the benzene ring.
Alkylation is a sort of Friedel Crafts reaction in which one of the benzene ring’s H atoms is replaced with an alkyl group.
The Friedel-Crafts reaction is an electrophilic aromatic substitution of an aromatic chemical. Two scientists, French Charles Friedel and American James Crafts, invented this well-known Friedel-Crafts reaction. In the Friedel-Crafts reaction, the aromatic molecule undergoes an electrophilic substitution, and the hydrogen atom in the benzene is substituted with an electrophile in the presence of a Lewis acid such as anhydrous aluminum chloride.
Interesting Science Videos
Friedel Craft alkylation
Friedel Crafts alkylation involves treating an alkyl halide with a Lewis acid in the presence of an aromatic ring. The alkyl group joins the ring, establishing a C-C bond, while the C-H bond is broken. Only an alkyl halide (chloride, bromide, iodide) should be used, not alkenyl, alkynyl, or aryl halides, or the reaction will fail. These species’ carbocations are exceedingly unstable and difficult to generate.
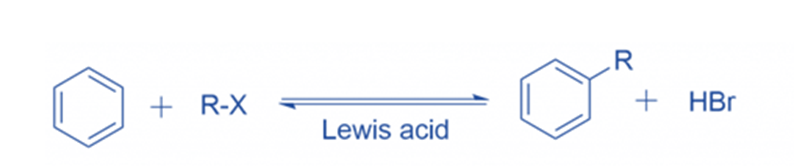
Reaction mechanism of Friedel Crafts alkylation
Generation of electrophile
Anhydrous aluminum chloride is a very helpful Lewis acid in the production of electrophiles for aromatic ring alkylation. Carbocations are formed in the presence of Lewis acid. The attacking reagent’s electron pair is accepted by the Lewis acid. R+ (carbocations)are generated from the conjunction of anhydrous aluminum chloride with the attacking reagent.

Formation of intermediate
When R+ attacks an aromatic ring, an arenium ion or sigma complex is formed. Another carbon in this arenium ion has gone through sp3 hybridization. This arenium ion achieves stability in a resonance configuration. Because electron delocalization occurs at the sp3 hybridized carbon,’ the aromatic property of the sigma complex or intermediate ion’ is lost.
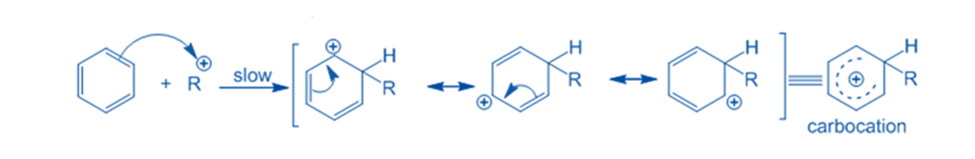
Abstraction of hydrogen
The sigma complex releases a proton from the sp3 hybridized carbon when it is attacked by AlCl4 to restore the aromatic characteristic. As a result, the electrophile replaces the hydrogen ion within the benzene ring. Because the concept of electrophilic substitution has been applied in the majority of organic name reactions, it is an extremely important reaction in organic chemistry.
Limitation of Friedel Craft alkylation
- Vinyl and aryl halides do not generate stable carbocations. As a result, they are ineligible for use in the Friedel-Crafts alkylation procedure.
- Aromatic rings with deactivating groups may not be ideal for Friedel-Crafts alkylation because the deactivating groups remove the electron density from the benzene ring, making a nucleophilic attack on the benzene ring impossible.
- The aniline amine group interacts with anhydrous aluminum chloride to generate a compound that deactivates the ring. As a result, the response is incomplete.
- Alkylation occurs on the benzene ring when it combines with an alkyl halide in the presence of anhydrous Lewis acid, however, polyalkylation may occur due to the activating nature of the alkyl group. To avoid this problem, a considerable amount of aromatic samples must be taken.
- The Lewis acid catalyst AlCl3 frequently binds with aryl amines, rendering them inactive.
- Isomerization and disproportionation can occur in the presence of excess catalysts and at high temperatures.
Friedel Craft acylation
An acylation reaction is one form of Friedel-Crafts reaction in which an RC=O group – an acyl group – is replaced by a hydrogen atom in the benzene ring.
Because benzene rings are relatively stable and unreactive, adding an acyl group can increase benzene’s reactivity.

In the presence of a Lewis acid catalyst such as AlCl3, this electrophilic aromatic substitution reaction occurs between arenes and acyl halides or anhydrides, resulting in the production of monoacylated compounds. The acyl halide’s halogen complexes with the Lewis acid generate a highly electrophilic acylium ion (RCO+), which becomes stable due to resonance. This reaction can only be utilized to generate ketones.
Mechanism of Friedel Craft acylation
Generation of electrophile
The Lewis acid catalyst (AlCl3) and the acyl halide undergo a reaction. A complex is formed, and the acyl halide loses a halide ion, resulting in the formation of an acylium ion that is stabilized by resonance.
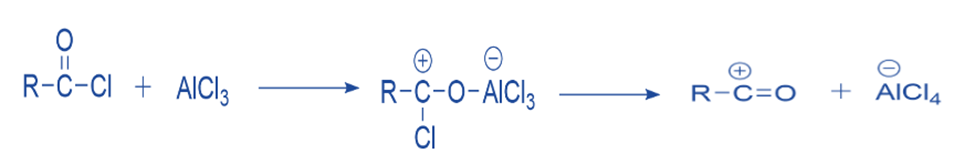
Formation of intermediate
The acylium ion attacks the ring electrophilically. As a result, the aromatic property of the ring is lost during complex formation.
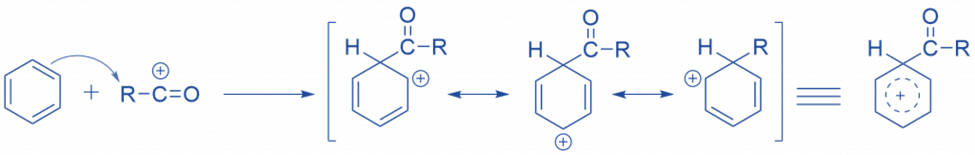
Abstraction of hydrogen
The intermediate complex is now deprotonated, restoring the ring’s aromaticity. A chloride ion extracts the proton from the complexed Lewis acid, creating HCl. The AlCl3 catalyst has been regenerated.
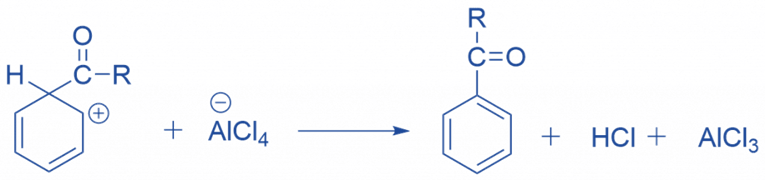
Limitation of Friedel Craft acylation
- Acylation can only produce ketones. Because of the reaction circumstances, HCOCl decomposes into CO and HCl.
- The Lewis acid catalyst AlCl3 frequently binds with aryl amines, rendering them inactive.
- Instead of the required ring acylation, amines, and alcohols can produce competing N or O acylations.
- Because they are the least reactive, compounds like mono halobenzenes do not respond or participate in the Friedel-Crafts acylation reaction.
Applications of Friedel-Crafts reactions
- Friedel-Crafts reactions are employed in the formation of C-C bonds.
- Friedel-Crafts reaction, i.e., alkylation process is mostly employed in industry to produce high-octane fuels, surfactants, fragrances, antioxidants, and other valuable chemicals such as cumene and thymol.
- Friedel Craft’s acylation is a major industrial procedure. It’s used to make chemical feedstock, synthetic intermediates, and fine compounds.
References
- Morrison R. T. & Boyd R. N. (1983). Organic chemistry (4th ed.). Allyn and Bacon.
- Smith M. & March J. (2001). March’s Advanced Organic Chemistry: Reactions Mechanisms and Structure (5th ed.). Wiley.
- Ghosh, S.K., Advanced General Organic Chemistry, Second Edition, New Central Book Agency Pvt. Ltd., Kolkatta, 2007.
- Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and Company Ltd, New Delhi, 1992. production of polyesters, polyurethanes, and alkaline resins.
