Gay-Lussac’s law, often known as one of the Ideal Gas Laws, establishes a relationship between the pressure of a gas and its absolute temperature, assuming that the volume remains constant. Gay-Lussac’s Law states that the pressure exerted by a gas is directly proportional to its temperature, assuming that the mass and volume of the gas remain constant. Gay-Lussac’s Law exhibits extensive applicability within the realm of scientific inquiry, as well as in several facets of our daily existence.
The scientific principle known as Gay-Lussac’s Law was formulated by the renowned French scientist Joseph Gay-Lussac in the year 1808.
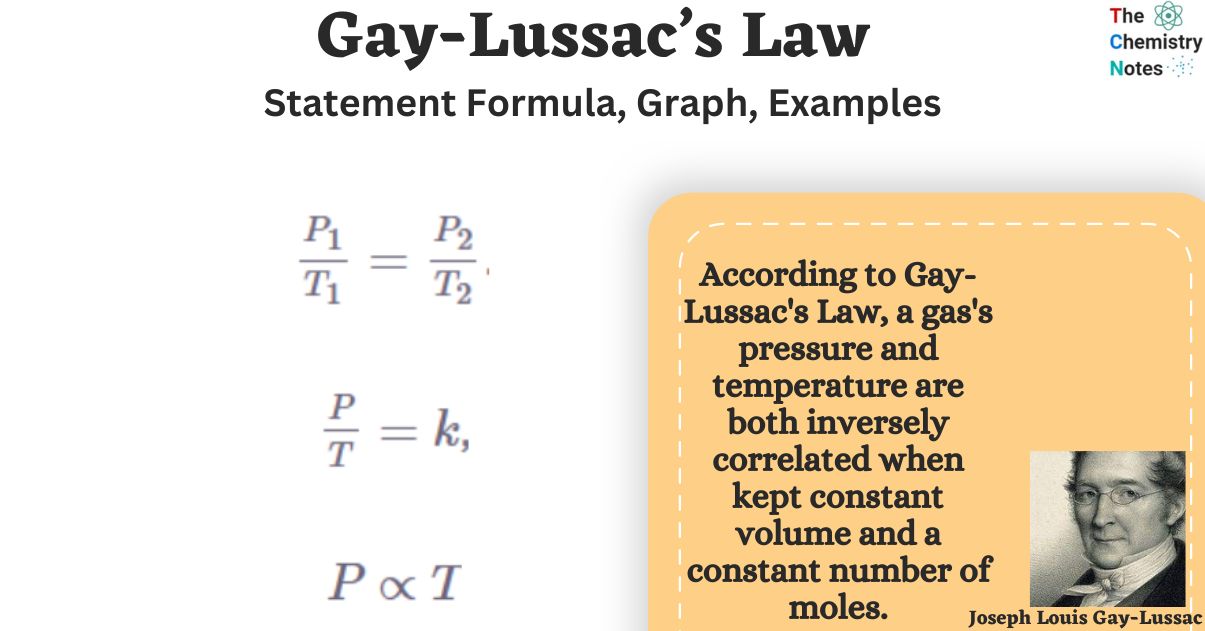
Interesting Science Videos
Who is Gay-Lussac?
French chemist Joseph Louis Gay-Lussac lived from 1778 to 1850. In the early 1800s, he discovered and shared his famous Gay Lussac’s law. He also developed numerous analytical chemistry techniques, discovered boron, and did much more besides. Humphry Davy, who discovered several other elements, including calcium and potassium, was his rival.
What is Gay-Lussac’s Law?
Gay-Lussac’s Law, sometimes known as the law of combining volumes, is a fundamental principle in the field of chemistry.
According to Gay-Lussac’s Law, a gas’s pressure and temperature are both inversely correlated when kept constant volume and a constant number of moles.
According to Gay-Lussac’s Law, temperature changes in response to changes in pressure.
Several mathematical representations exist for Gay-Lussac’s Law mentioned below:
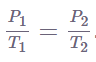
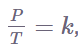
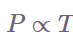
Where:
- P is the pressure exerted by the gas
- T is the absolute temperature of the gas
- k is a constant.
Gay-Lussac’s Law Equation Derivation
Gay-Lussac’s law establishes a mathematical expression that describes the relationship between pressure and temperature, under the condition that volume and mass/moles remain constant. This can be interpreted as:
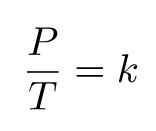
Since they are both equal to the same constant number, we can also relate pressure and temperature at two different points.
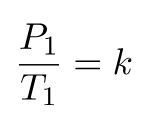
And
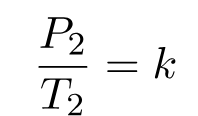
Therefore,
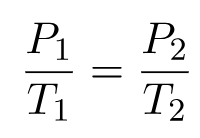
The formula may be observed in various ways.
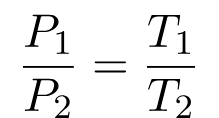
The value of k in these equations can also be determined by rearranging the ideal gas law.
We are maintaining constant volume (V) and moles (n). The entire right-hand side of the lower equation represents a constant value.
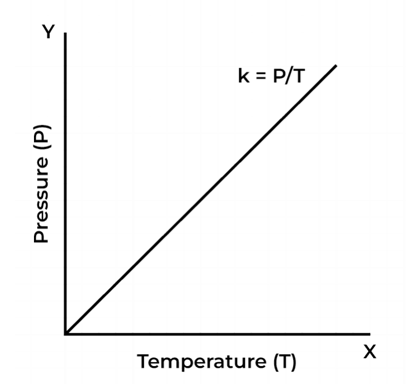
Gay Lussac’s Law Graph
The equation represents the law of Gay Lussac.
P = kT
This equation is mathematically comparable to y = mx
Comparing equations we obtain
- x = T
- y = P
- m = k
Practical Applications of Gay-Lussac’s Law
Some commonplace applications of Gay-Lussac’s law are listed below.
Automobile Tire
Automobile tire pressure decreases on cold days and increases significantly on hot days. When tires get hot, the air inside of them expands, so it’s important not to over-inflate them when they’re cold. In a similar vein, your tires will be underinflated while cold even if they show the correct pressure when hot.
Pressure Cooker
In a pressure cooker, the internal pressure is raised through the use of heat. The boiling point of water is increased by applying pressure, which allows for faster cooking. Flavors are preserved since no steam may escape the airtight container.
Aerosol Cans
Storing or disposing of aerosol cans in hot environments or burning them raises the pressure of the contents, which could cause the can to rupture.
Electric Water Heaters
Electric water heaters are very similar to pressure cookers in operation. The buildup of steam can be avoided with the help of a pressure-relief valve. The pressure of the steam inside the heater will increase if the valve fails.
Gay Lussac’s Law of Gaseous Volumes
The law of Gaseous Volumes, also proposed by Joseph Louis Gay-Lussac in 1808, is distinct from the law of definite proportions. If the volumes of the interacting gases are determined at the same temperature and pressure, then the ratio of the volumes is a tiny whole number, as stated by Gay Lussac’s Law of Gaseous Volumes. In contrast to the law of definite proportion, which applies to the gas’s mass, Gay Lussac’s Law of Gaseous Volumes is concerned only with the gas’s volume.
For Example:
N2 + 3H2 → 2NH3
1 volume of N2 reacts with three volumes of H2 to give 2 volumes of NH3
Frequently Asked Questions
What is the importance of Gay Lussac’s law?
Assuming no change in volume, the meaning of this gas law is to show that a rise in temperature results in a corresponding increase in pressure. Likewise, the strain can be reduced in direct proportion to the temperature drop.
What is Gay Lussac’s law formula?
The law of Gay-Lussac is a special case of the ideal gas law in which the volume of the gas is fixed. A gas’s pressure increases linearly with its temperature, provided that the gas’s volume remains constant. In most cases, Gay-Lussac’s law is calculated using the constant value of P / T or the formula P1/ T1 = P2 / T2.
What is the difference between Gay Lussac’s Law and Charles’s Law?
When compared to Charles’ Law, Gay Lussac’s Law primarily differs in that,
At constant pressure, the volume of a gas is proportional to its absolute temperature per Charles’s law, but under Gay-Lussac’s Law, the pressure is proportional to the temperature for a given gas volum
Video on Gay-Lussac’s Law
References
- https://chemistrygod.com/gay-lussac-law-examples
- Crosland, M. P. (1961). “The Origins of Gay-Lussac’s Law of Combining Volumes of Gases”. Annals of Science, 17 (1): 1. doi:10.1080/00033796100202521
- https://sciencenotes.org/gay-lussacs-law-definition-formula-examples/
- https://byjus.com/chemistry/gay-lussacs-law/
- https://chemistrytalk.org/gay-lussacs-law/
- https://www.expii.com/t/gay-lussacs-law-overview-formula-11095
- https://unacademy.com/content/neet-ug/study-material/chemistry/gay-lussacs-law/
- https://www.geeksforgeeks.org/gay-lussacs-law/

