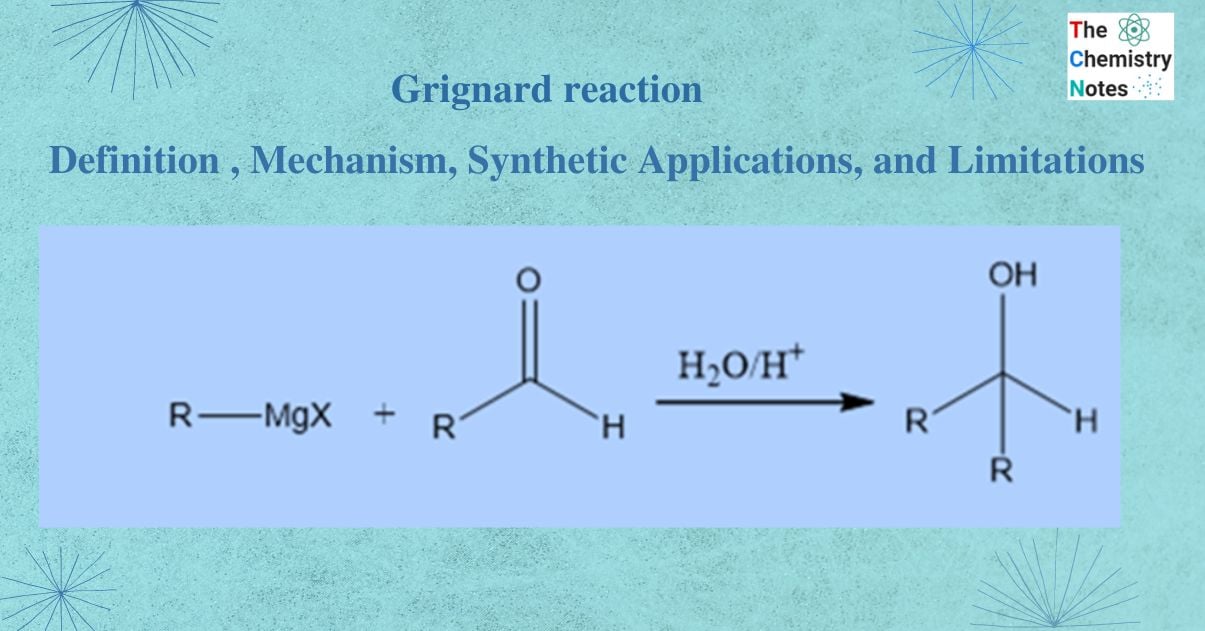
Grignard reaction describes the addition of alkyl magnesium halides to the carbonyl group of aldehyde or ketone.
This reaction is one of the most significant process for the formation of carbon-carbon bonds. In this reaction process alkyl, vinyl, or aryl magnesium halides are regarded as Grignard reagents. The Grignard reactions and reagents are named after the French scientist Francois Auguste Victor Grignard, who made the discovery and was honored with the 1912 Nobel Prize in Chemistry.
Grignard reagent
An alkyl bromide and magnesium metal react to create an organomagnesium reagent in the first step of a Grignard synthesis. The resulting “Grignard reagent” serves as a potent base as well as a powerful nucleophile. As a result of its nucleophilic nature, it can interact with the electrophilic carbon in a carbonyl group to form the carbon-carbon bond.
Because of its basic nature, it will react with acidic substances such as alcohols, water, phenols, carboxylic acids, and thiols. As a result, the reaction environment must be absolutely anhydrous and free of acids. Grignard reagents can also create hydroperoxides when they interact with oxygen. So they are quite unstable when exposed to the air.
Interesting Science Videos
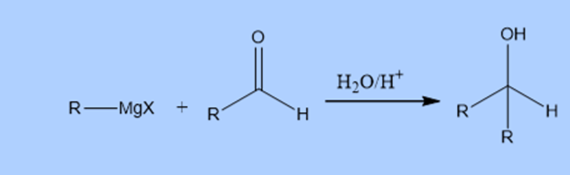
Reaction mechanism of Grignard reaction
Firstly, nucleophilic attack alkyl or aryl group of Grignard reagent on the carbonyl group of carbonyl compounds to give intermediate complex. Which on hydrolysis produces corresponding alcohols.
Some synthetic applications of Grignard reaction (reagent)
1. Alkane formation
The Grignard reagent reacts with water, alcohol acid, and amine to give corresponding hydrocarbons.
E.g., Propylmagnesiumbromide produces propane gas when treated with water.
CH3 – CH2 – CH2 Mg Br + H2O → CH3 – CH2 – CH3 + Mg (OH) Br
When the Grignard reagent is treated with an alkyl halide, a coupling reaction occurs to give alkane. This reaction is catalyzed by the cuprous ions.
E.g., Methyl magnesium bromide reacts with methyl bromide to produce ethane.
CH3 – MgBr + CH3Br → CH3 – CH3 + MgBr2
2. Formation of acid
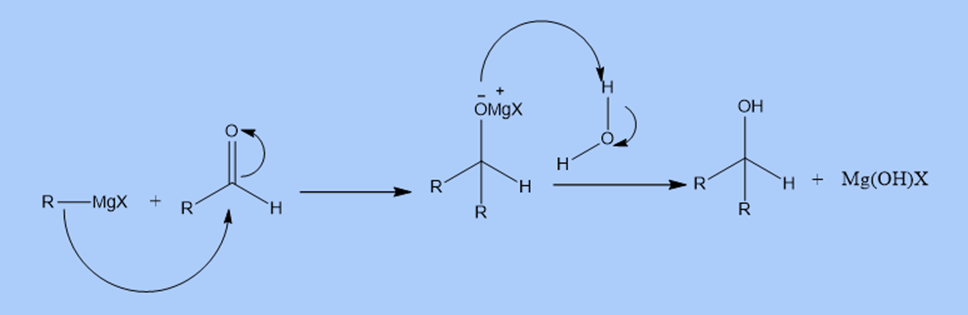
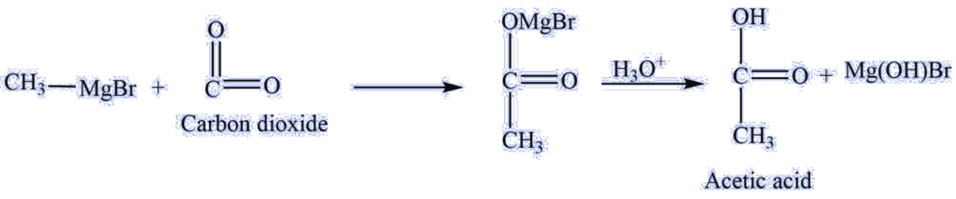
a. The Grignard reagent reacts with carbon dioxide to give carboxylic acid.
For example., methylmagnesium bromide reacts with carbon dioxide to give acetic acid.
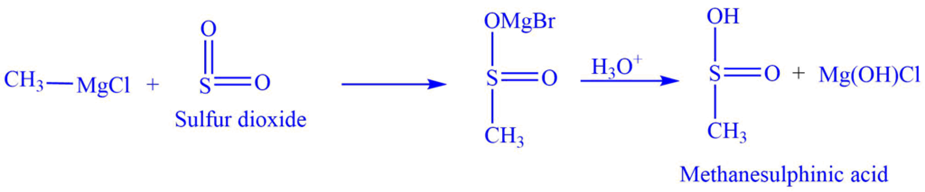
b. Reaction of the Grignard reagent with Sulfur dioxide SO2 to produce alkane sulphinic acid.
For example, reaction of methylmagnesium chloride with sulfur dioxide, SO2 produces methanesulphinic acid.
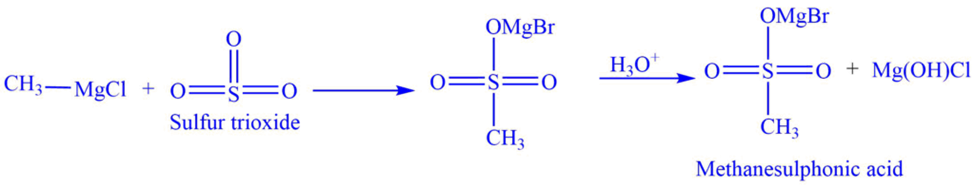
c. The Grignard reagent reacts with sulfur trioxide (SO3), to give alkane sulphonic acid.
E.g., Methanesulphonic acid is formed when methylmagnesium chloride reacts with sulfur trioxide (SO3).
3. Preparation of ketone
Nitriles, in reaction with the Grignard reagent, produce Ketone. However, addition of the Grignard reagent with hydrogen cyanide produces the aldehyde.
E.g., Acetonitrile, for example, produces acetone when reacted with methyl magnesium iodide.
CH3 – MgI + CH3 – CN → (CH3)2 C = N -MgI + H2O → CH3 – CO – CH3 + Mg (OH) I + NH3
CH3 – MgI + H – CN → CH3CH = N -MgI + H2O → CH3 – CO – H + Mg (OH) I + NH3
4. Other preparative reactions
1. Reaction of Chloramine, and NH2Cl with a Grignard reagent to produce amines.
CH3 – MgX + Cl – NH2 → CH3 – NH2 + MgXCl
2. Nitriles can be prepared by the reaction of Grignard reagents with cyanogen or cyanogen chloride.
CH3 – MgX + NCCl (cyanogen chloride) → CH3 – CN + MgX(CN)
3. Grignard reagents can be used to prepare alkyl iodides. Iodine reacts with alkyl magnesium chlorides or bromides to produce the corresponding alkyl iodides.
CH3 – MgBr + I2 → CH3 – I+ MgICl
Limitations of Grignard reaction
The reaction environment must be absolutely anhydrous and free of acids.
References
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987.
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Skyes, P., A Guide Book to Mechanism in Organic Chemistry, Second edition, Orient Longman Ltd., 1988.
