Hassium is a synthetic transition metal with an atomic number of 108 and is represented by the symbol ‘Hs’ in the periodic table. It is silvery in appearance and belongs to the d-block of period 7 of the periodic table. Hassium was the fourth transactinide (super-heavy) element identified. Only tiny quantities of bohrium have been successfully synthesized, hence there isn’t much known about it based on experimental data, but some qualities can be predicted using periodic table trends.
Hassium was synthesized in 1984 by Peter Armbruster, Gottfried Munzenber, along with others in GSI at Darmstadt, Germany. The GSI team attacked a lead-208 target using iron-58 nuclei.
Interesting Science Videos
Discovery and History of Hassium
- Hassium was known as eka-osmium or as element-108 according to Mendeleev’s nomenclature of unidentified elements.
- The effort to synthesize hassium began in 1978, with a team of scientists led by Yuri Oganessian at the Joint Institute for Nuclear Research (JINR) in Dubna, Russia. They didn’t get the expected results.
- Hassium was synthesized in 1984 by Peter Armbruster, Gottfried Munzenber, along with others in GSI at Darmstadt, Germany. The GSI team attacked a lead-208 target using iron-58 nuclei.
- The element was named after Hassia, the Latin name for the German state of Hesse, where the GSI Institute was based.
- In 1992, Peter Armbruster came up with the name for the element. Hassium was approved as the designation by the International Union of Pure and Applied Chemistry in 1997. The letter Ha is its symbol.
Occurrence of Hassium
- Hassium is a manmade element that does not exist naturally. Approximately one hundred atoms of hassium have been synthesized to yet.
- Hs is a radioactive metal generated through nuclear bombardment. It has only been manufactured in minute quantities. Hassium is created by blasting 208Pb with 58Fe.
- It contains 12 isotopes having known half-lives ranging in mass from 263 to 277.
- Hs was initially synthesized by cold fusion of lead-208 with iron-58 nuclei using the following procedure.
208Bi + 58Fe → 265Hs + 1n- It decays quickly.
- Victor Cherdyntsev, a Russian chemist, also claimed to have discovered Hassium occurring naturally. However, he was unable to corroborate his statement.
Elemental Properties of Hassium
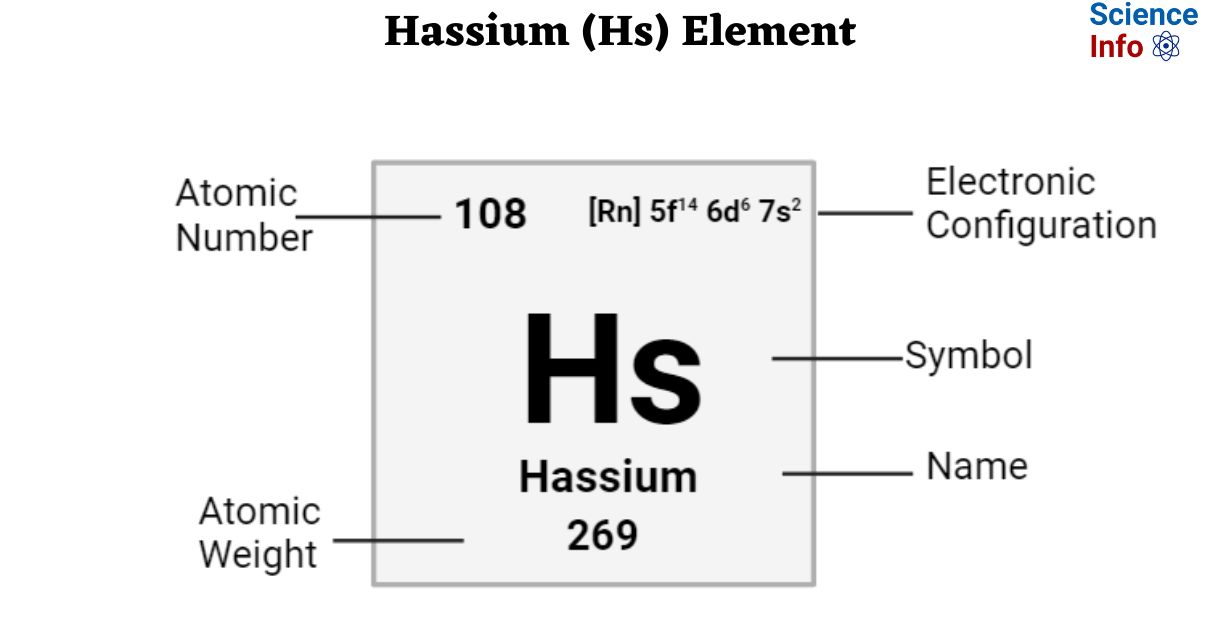
| Electronic Configuration | [Rn] 5f14 6d6 7s2 |
| Atomic Number | 108 |
| Atomic Weight | 269 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | Trans-actinides, 7, d-block |
| Density | unknown |
| Ionic radius | – |
| Van der Waals radius | – |
| Electron shells | 2, 8, 18, 32, 32, 14, 2 |
| Electrons | 108 |
| Protons | 108 |
| Neutrons | 161 |
Isotopic Information of Hassium
- Hassium has no stable isotopes naturally, but they can be created in a laboratory setting.
- All of the hassium isotope are unstable and radioactive.
- Multiple radioactive isotopes have been created in the lab, either by fusing two atoms or by studying the decay of heavier elements.
- Given the short half-lives of the known hassium isotopes, no primordial hassium could have persisted to the present day.
- However, it is feasible that nuclear isomers or isotopes with longer half-lives may be discovered in trace amounts.
- Hassium contains 12 isotopes, with masses ranging from 263 to 277.
- The atomic number 108 is a proton magic number for deformed (non-spherical) nuclei, whereas 162 is a neutron magic number for them.
- This doubly magic nucleus has a lower decay energy than other hassium isotopes.
- The most stable isotope is Hs-269, with a half-life of 9.7 seconds.
- Hs-270 is particularly interesting since it has a “magic number” of nuclear stability.
- More research is required to determine whether Hs-270 is an isotope in the proposed island of stability.
Physical Properties of Hassium
- The instability of hassium makes it difficult to conduct a statistically significant investigation of its physical properties.
- Due to its rapid disintegration, only a few properties of hassium have been investigated until now.
- Hassium is a synthetic, radioactive, and super-heavy transactinide element. It is expected to be solid under normal conditions.
- It is found in the 7th period, the 8th Group, and the d-block of the periodic table.
- The melting point and the boiling point of the element 108 is yet to be known however the boiling point of the element is predicted to be high.
- The atomic mass of hassium is 269.
- The density of hassium is also unknown as of now.
- According to periodic law, hassium should be the heaviest element in group 8 of the periodic table.
- Hassium crystallizes in the hexagonal close-packed structure (hcp), and its bulk modulus (compression resistance) is comparable to diamond (442 GPa).
- The longest-lived hassium isotope has a half-life of almost ten seconds.
Chemical Properties of Hassium
- The instability of hassium makes it difficult to conduct a statistically significant investigation of its chemical properties.
- The features of hassium that have been investigated are solely connected to gas-phase chemistry. The majority of the attributes are purely theoretically determined.
- As a transition metal, hassium is predicted to possess characteristics comparable to those of the platinum group of metals. According to predictions, it will chemically mirror iron, ruthenium, and osmium.
- Hassium’s anticipated oxidation states based on its electrical configuration are +8, +6, +4, +3, and +2.
- The most stable oxidation state for hassium has been proposed as +8.
- It is expected to react rapidly with oxygen(O), producing a highly volatile tetroxide.
Synthesis of Hassium
- All elements with atomic numbers more than 100 can only be created through reactions in a particle accelerator, such as a cyclotron; they do not develop in a nuclear reactor.
- When Lead (Pb)-208 blasted with ferrous-54 it produces hassium-264.
Uses of Hassium
- Given barely any atoms of this metal have been created to date, there are currently no specific or exclusive uses of Hassium outside of scientific research.
- Furthermore, because it is unavailable in nature, Hassium is only employed by scientific researchers, with no recognized negative effects or uses for the metal among individuals and organizations.
- Hassium is currently solely used in studies to learn more about its characteristics and to manufacture isotopes of other elements.
- Hassium performs no biological purpose. Because it is a heavy metal that decays to release alpha particles, it is highly poisonous.
Health Effects of Hassium
- Hassium is a very unstable chemical; when created, it swiftly decomposes into other elements, therefore it does not influence human health.
Environmental Effects of Hassium
- Hassium’s environmental ramifications are unnecessary due to its relatively short half-life (about 10 seconds).
Video Reference
References
- https://www.rsc.org/periodic-table/element/108/hassium
- https://chemicalengineeringworld.com/hassium-element-properties-and-information/
- https://www.thoughtco.com/hassium-facts-4136901
- https://www.chemistrylearner.com/hassium.html
- https://www.chemicool.com/elements/hassium.html
- https://periodic-table.com/hassium/
- https://www.vedantu.com/chemistry/hassium