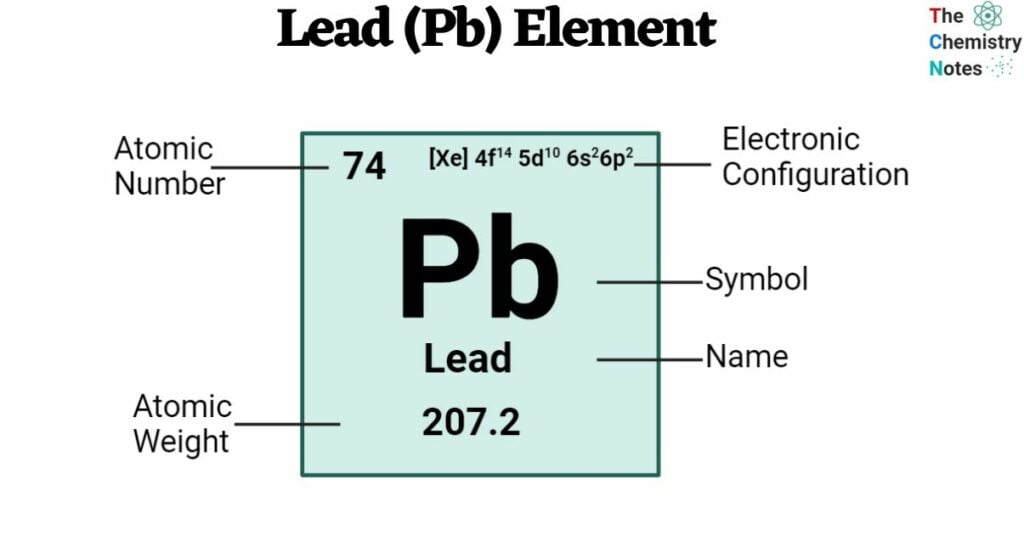
The atomic number of Lead is 82. It is found in Group 14 (carbon group) and Period 6 of the periodic chart. The symbol ‘Pb’ represents it. It gets its name from the Anglo-Saxon word “lead,” and the Latin word “plumbum,” which means “waterworks,” is the basis of its symbol, Pb. Lead occurs seldom in nature and may be found in ores, primarily alongside copper, zinc, and silver. The main lead mineral is lead sulfide (galena, PbS).
Lead compounds are often found in the +2 oxidation state, rather than the +4 state seen in lighter members of the carbon group. Organolead compounds are the only exceptions.
Interesting Science Videos
History of Lead
- Early evidence of metal smelting can be documented in the form of metallic lead beads discovered in Asia Minor between 7000 and 6500 BC.
- The use of lead minerals in cosmetics dates back to the Ancient Egyptians, which then eventually spread to Ancient Greece and other civilizations. In their fishing nets, glazes, glasses, enamels, and decorations, the Egyptians may have utilized lead as sinkers.
- Lead was utilized by several cultures in the Fertile Crescent for building, currencies, and inscriptions.
- The ancient Chinese royal court employed lead as a stimulant, a kind of money, and a method of contraception.
- Eastern and southern Africans, Mesoamericans, and Indus Valley Civilization people made amulets out of wire drawn from lead.
Occurrence of Lead
- About 0.0013% of the earth’s crust is made up of natural Pb, which may also be found in a variety of mineral ores such as anglesite, galena, and cerussite.
- Its most common ore is galena, also known as lead sulfide (PbS). Anglesite (PbSO4) and mimetite (Pb5(AsO4)3Cl are two more lead ores.
- Heat is used to purify lead from galena (PbS). Recycling often retrieves a significant proportion of lead.
- Lead has 35 isotopes with known half-lives, with mass numbers ranging from 181Pb to 215Pb. Lead occurs naturally as a combination of four isotopes.
Isotopes of Lead
Lead consists of Four naturally occurring isotopes: 204Pb, 206Pb, 207Pb and 208Pb.
| Isotopes | Natural abundance (atom %) |
|---|---|
| 204Pb | 1.4 |
| 206Pb | 24.1 |
| 207Pb | 22.1 |
| 208Pb | 52.3 |
Elemental Properties of Lead
| Electronic Configuration | [Xe] 4f14 5d10 6s2 6p2 |
| Atomic Number | 82 |
| Atomic Weight | 207.2 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | 14, 6, p-block |
| Density | 11.34 g/cm3 at 20 °C |
| Ionic radius | 0.132 nm (+2) ; 0.084 nm (+4) |
| Van der Waals radius | 0.154 nm |
| Electron shells | 2, 8, 18, 32, 18, 4 |
| Electrons | 82 |
| Protons | 82 |
| Neutrons | 126 |
Physical Properties of Lead
- Lead has an atomic number of 82 and is a grey metal. It has a melting point of 327.46 °C(621.43 °F) and a boiling point of 1749 °C (3180 °F).
- Pb has a solid phase density of 11.34 g/cm3 and a liquid or molten phase density of 10.66 g/cm3.
- Freshly cut lead appears silver with a tinge of blue.
- Pb is malleable meaning it can be easily hammered into sheets without any cleavage.
- It is also ductile metal which can be drawn into thin wires without breaking it.
- Pb is simple to deal with. “Working” a metal entails bending, cutting, shaping, tugging, and generally modifying its shape.
- It tarnishes slowly in humid air, forming a dull gray coating.
- It is exceedingly resistant to corrosion and a poor conductor of electricity, sound, and vibration.
| Color/physical appearance | Metallic grey |
| Melting point/freezing point | 600.61 K (327.46 °C, 621.43 °F) |
| Boiling point | 2022 K (1749 °C, 3180 °F) |
| Density | 11.34 g/cm3 at 20° |
| Malleability | Yes |
| Ductility | Yes |
| Electronegativity | 2.33 (in +4), 1.87 (in +2) (Pauling Scale) |
Chemical Properties of Lead
- Lead compounds have two distinct oxidation states: +4 and +2.
- It disintegrates slowly in water and most cold acids.
- Lead metal is resistant to phosphoric and sulfuric acids, but it is vulnerable to nitric acid (HNO3) and hydrochloric acids.
- It does not easily react with oxygen in the air and does not burn.
- During the reaction with chlorine, it must be heated.
- In exposure to moist air, lead tarnishes rather quickly.
- When the metal is burned in the air in its powdered state, a bluish-white flame results.
- Lead and fluorine react swiftly at room temperature.
Chemical Reaction of Lead
- The Reaction of Lead with Air
The surface of metallic lead is protected by a thin layer of lead oxide (PbO). Only when lead is heated to 600-800 °C does it react with oxygen in the air to produce lead oxide, PbO.
2 Pb (s) + O2 (g) ⇌ 2 PbO (s)- The Reaction of Lead with Water
In the lack of air, lead does not react with water. Lead(II) hydroxide is created in the presence of air.
2 Pb (2) + 2 H2O (l) + O2 (g) → 2 Pb(OH)2 (s)- The Reaction of Lead with Halogens
When heated, lead metal reacts strongly with chlorine, Cl2, to generate lead(II) chloride, PbCl2.
when heated Δ
Pb (s) + Cl2 (g) → PbCl2 (s)At normal temperatures, the hazardous dihalides lead(II) fluoride, PbF2, are formed when lead metal and fluorine, F2, react strongly.
Pb (s) + F2 (g) → PbF2 (s)- The Reaction of Lead with Acids
Because of the passivated PbO surface, lead does not react with sulfuric acid.
Lead reacts slowly with hydrochloric acid and nitric acid, HNO3.
Pb (s) + 2 HCl (aq) ⇌ Pb2+ (aq) + 2 Cl− (aq) + H2 (g)Nitrogen oxides are generated in conjunction with lead(II) nitrate, Pb(NO3)2.
Pb (s) + 2 HNO3 (aq) ⇌ Pb2+ (aq) + 2 NO3− (aq) + H2 (g)Uses of Lead
- Lead is utilized in roofing materials, roof parapets, flashing, cladding, gutters, and gutter joints.
- It is utilized in pencils, flashings, lifting weights, anchors, and diving weight belts.
- As a corrosion-resistant metal, it is used in the production of pipes, paints, pesticides, and hair colors. It is also added to gasoline as an anti-knocking agent to improve engine performance.
- Because of its great density, formability, and atomic number, lead may be used as a sound, vibration, and even radiation barrier. Furthermore, lead has no resonant frequencies.
- Lead-sheathed cables and wires used in petrochemical facilities serve as effective chemical barriers against hydrocarbons and moisture.
- Its oxide-coated glasses are used to make windows (known as leadlight) and roofing. Pb crystal glasses are occasionally used to store corrosive liquids.
- Pb may be alloyed with other metals to create a wide range of pigments used in paints, road marking, and galvanized steel plating.
- Nuclear reactors and X-ray equipment with lead coatings aid in the reduction of dangerous X-rays and gamma-rays.
- The most popular application for the metal is in lead-acid batteries. Sulfuric acid, lead, and lead oxide may all be used for generating electricity efficiently.
Health Effects of Lead
- Lead may enter drinking water via pipe corrosion. Whenever the water is slightly acidic, this is more probable to happen. As a result, municipal water treatment facilities are now obligated to perform pH changes in drinking water.
- Lead may enter a fetus through the mother’s placenta. As a result, it has the potential to seriously harm unborn children’s nerve systems and brains.
- Physical: an increase in blood pressure, renal damage, brain and nervous system damage, sperm destruction, and miscarriage
- Psychological: reduced learning ability, hostility and impulsive conduct, and hyperactivity
Environmental Effects of Lead
- Some creatures, including as selfish and phytoplankton, are poisoned by lead even at low amounts. Phytoplankton is a major source of oxygen generation in the seas.
- Lead may build up in the food chain due to soil poisoning, which affects soil organisms.
Video References
References
- https://www.lenntech.com/periodic/elements/pb.htm
- https://byjus.com/chemistry/lead/
- https://www.chemicool.com/elements/lead.html
- https://www.chemistrylearner.com/lead.html
- https://chemicalengineeringworld.com/lead-element-properties-and-information/
- https://pilgaardelements.com/Lead/Reactions.htm
- https://www.webelements.com/lead/chemistry.html#:~:text=Lead%20metal%20reacts%20vigorously%20with,chloride%2C%20PbCl2%2C%20respectively.

