Hell Volhard Zelinsky reaction is a halogenation reaction that involves the halogenation of carboxylic acids at the α carbon.
The reaction is named after the German chemists Carl Magnus von Hell and Jacob Volhard and the Russian chemist Nikolay Zelinsky (1861– 1953).
When aliphatic acids with at least one alpha-hydrogen react with bromine in the presence of phosphorus tribromide, alpha-bromoacid is produced. This reaction is known as the Hell Volhard Zelinsky reaction.
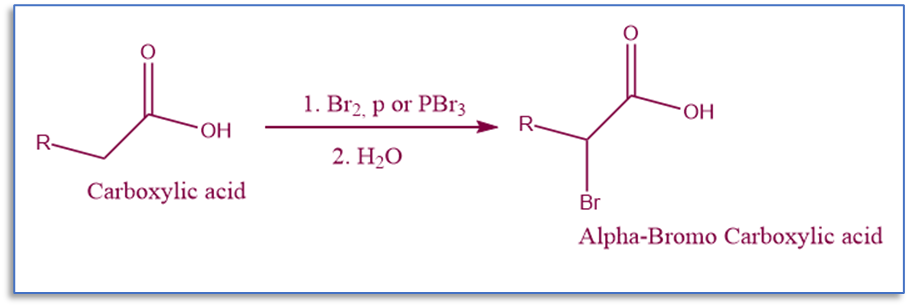
Simply, this is the alpha-halogenation of carboxylic acid utilizing X2 and PX3. In addition, acyl halides or anhydrides, which are acid derivatives, can be halogenated without the use of a catalyst. Despite having several benefits, this process fails to fluorinate or iodinate carboxylic acids.
The reaction mechanism differs from other halogenation reactions in that it occurs in the lack of a halogen carrier. It needs particular reaction circumstances. When this reaction is carried out at very high temperatures, hydrogen halide may be eliminated from the product, leading to the synthesis of beta unsaturated carboxylic acids.
Interesting Science Videos
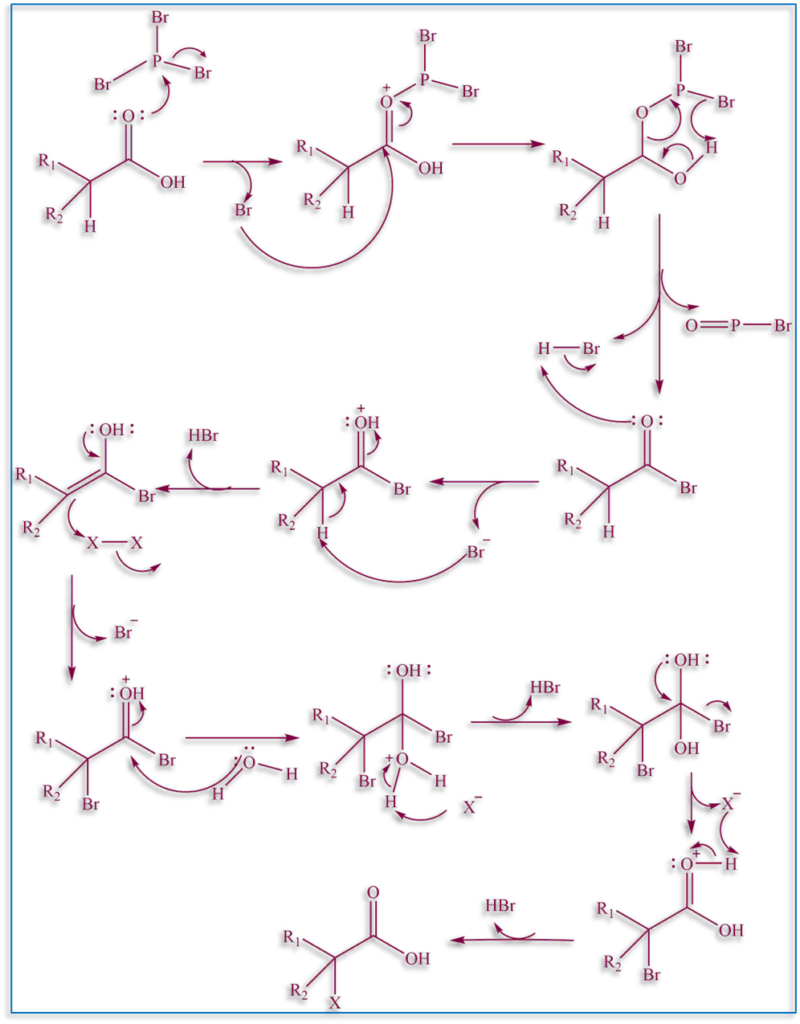
Reaction Mechanism
The formation of a P-O bond and release of a halide anion occurs as the first step in the mechanism when carbonyl oxygen reacts with phosphorous trihalide. After attacking the carbonyl, the halide forms an intermediate . The intermediate rearranges to produce an acyl halide, an acid molecule, and a phosphine oxide. The enol form of the acyl halide then attacks the halogen molecule to generate a -halo acyl halide when the acyl halide tautomerizes to the enol form. The end product, a -halo carboxylic acid, is produced by water hydrolysis.
Applications
- Generally, it is used for the halogenation of carboxylic acid at the alpha position.
- Amino acid synthesis can also be carried out via the Hell -Volhard-Zelinsky process. Amino acids are produced by reacting the generated alpha-Bromo acid with excessive ammonia.
- Alpha cyano acid, which is produced by reacting alpha bromoacid with cyanide ions, can also be hydrolyzed to create malonic acid.
Limitations
- Acids without alpha hydrogen do not undergo this reaction.
References
- Skyes, P., A Guide Book to Mechanism in Organic Chemistry, Second edition, Orient Longman Ltd., 1988
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987.
