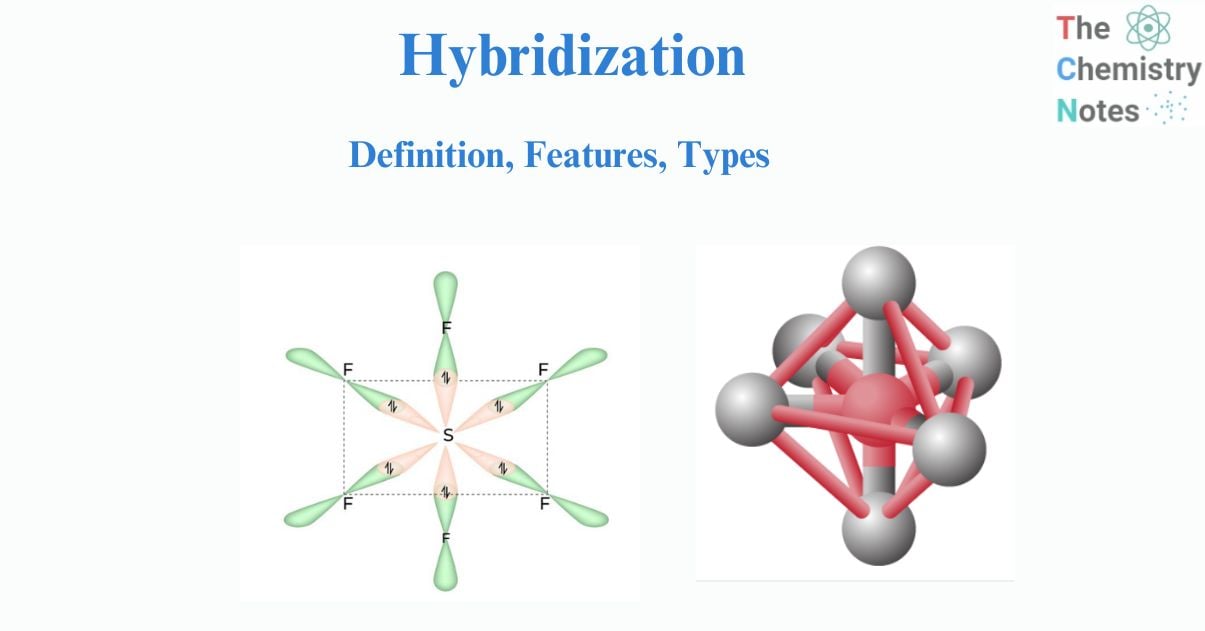
Hybridization is the process of combining two or more non-equivalent atomic orbitals with similar energies but different forms. The process of combining two atomic orbitals to form a new type of hybridized orbitals is termed hybridization. Atomic orbitals of the same energy level undergo hybridization to form a new set of orbitals with equivalent energy and equivalent shape.
The new orbitals created as a result of this process are known as hybrid orbitals. More crucially, hybrid orbitals can be utilized to explain the properties of atomic bonds and molecular geometry. The energy of these orbitals is always less than the energy of the parent orbitals. As a result, hybrid orbitals are more stable than parent atomic orbitals. Hybridization is a valence bond theory extension that helps us understand bond formation, bond energy, and bond lengths.
Interesting Science Videos
What is hybridization?
Hybridization is the process of combining atomic orbitals of comparable energy to form new orbitals with equivalent energy. The new orbitals that result from this process are known as hybrid orbitals.
According to the quantum mechanical approach, the wave functions of the atomic orbitals of a central atom are combined in such a way that electron densities are redistributed. As a result, new electron clouds are created that are mutually identical to each other. These new electron clouds (hybrid orbitals) establish the strongest possible bonds and dictate a molecule’s shape.
The necessity of the concept of hybridization
The formation and stereochemistry (geometry) of a large number of polyatomic molecules cannot be explained by the simple concept of atomic orbital overlapping. Let us take the formation of CH4
molecule for example, the Valence shell electronic configuration of C-atom is 2s2, 2px1, 2py1 It has just two unpaired electrons on the valence shell. For the formation of four covalent bonds, the C-atom undergoes excitation, which promotes one electron from the 2s orbital to the 2pz orbital.
So electronic configuration in the excited state becomes: 2s2, 2px1, 2py1, 2pz1
The C-atom possesses one s-orbital electron and three p-orbital electrons. If we anticipate constructing a CH4 molecule by overlapping pure atomic orbitals, three bonds should be formed by overlapping the 1s orbital of H and the 2p orbitals of the C-atom, and one bond should be made by overlapping the Is orbital of H and the 2s orbital of carbon.
Furthermore, the C—H bond generated by the Is – 2s overlap should be weaker than the other three bonds formed by the Is — 2p overlap. The experimental results reveal that all four bonds in CH4 are comparable and have the same bond angle, 109. 50. To address these issues, a hypothetical concept of hybridization was created to explain why all bonds in a molecule are (essentially) equivalent. The explanation of molecular geometry is the most significant use of this idea.
Therefore, the fundamental idea behind hybridization is that in polyatomic molecules, the central atom cannot directly form covalent bonds with other atoms using its atomic orbitals. Rather, before establishing bonds, the central atom mixes the valence shell orbitals to make new types of orbitals with equivalent energy. And, when these new orbitals (known as hybrid orbitals) combine to form a molecule with identical bonds.
Linus Pauling proposed the hybridization phenomenon to explain molecular geometries. He pointed out that bonding in methane molecules involves four atomic orbital carbon atoms. That explains why methane lacks a pi-bond distribution.
Methods for Determining the Type of Hybridization
The following guidelines must be followed in order to understand the type of hybridization in an atom or an ion.
- First, calculate the total number of valence electrons in an atom or ion.
- Then, count how many lone pairs are linked to that atom or ion.
- The number of orbitals necessary can now be determined by adding the number of duplex or octet electrons and the number of lone electron pairs.
- The shape of orbitals in atoms or ions changes when there isn’t a lone pair of electrons, which should be highlighted.
Features of Hybridization
- The atomic orbitals of the central atom’s valence shell are involved in hybridization.
- The atomic orbitals involved in hybridization should have comparable energy.
- The number of hybrid orbitals equals the number of mixed atomic orbitals.
- There are three different kinds of atomic orbitals that can participate in hybridization:
(a) orbitals with unpaired electrons.
(b) Atomic orbitals containing both unpaired and paired electrons.
(C) Atomic orbitals that are not occupied.
- Hybrid orbitals usually form an σ -bond. It will never create a π-bond.
- The atomic orbitals required to produce π -bonds are not hybridized. In other words, the atomic orbitals that must form σ -bonds are exclusively involved in hybridization.
- A hybrid orbital, like an atomic orbital, is able to accommodate two electrons.
- The shape of hybrid orbitals is independent of the intermixing atomic orbitals.
- A hybrid orbital consists of two lobes, one larger and one smaller. Only the bigger lobes take part in bonding.
- Because the electron density in the hybrid orbital is concentrated in the bigger lobe, the extent of overlap between the hybrid orbital and the atomic orbital is maximum. As a result, hybrid orbitals establish strong bonds.
- All hybrid orbitals are degenerate, which means they all have the same energy and shape.
- The hybrid orbitals are oriented spatially around the center atom. The spatial orientation (stereochemistry) is determined by the number of hybrid orbitals, which is the total of σ -bond pairs and lone pairs.
- The type of hybridization involved in the center atom determines the shape of a molecule. The type of hybridization is determined by the number of hybrid orbitals, which is the total of σ -bond pairs and lone pairs on the valence shell of the central atom.
Types of hybridization
The types of hybridization are determined by the number and kind of atomic orbitals involved. There are various kinds of hybridization depending on the orbitals involved in the mixing, such as sp, sp2, sp3, sp3d, sp3d2, and so on.
sp hybridization
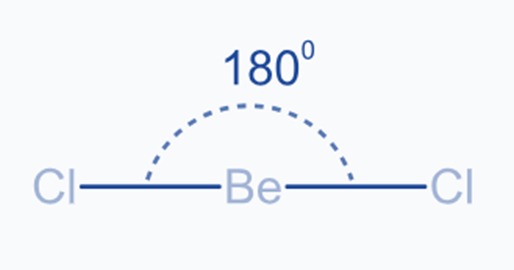
This type of hybridization involves the atom’s s subshell orbital and p subshell orbital being mixed to generate two sp-hybridized orbitals with equivalent shapes and energy. sp hybridized orbitals arranged in space at a 180° angle. This type of hybridization is also known as diagonal hybridization. Each sp hybrid orbital has an equal amount of s and p characters, i.e., 50% s and 50% p. Linear molecules have an sp hybridized center atom that is directly bonded to two other atoms.
All beryllium compounds including BeF2, BeH2, and BeCl2 are examples of sp hybridized molecules.
For example:
Hybridization of BeCl2
ground state configuration of Be is 1s2, 2s2.
in the excited state one of the 2s electrons is promoted to 2p orbitals. So,
The excited state configuration of Be is 1s2, 2s1, 2px1, 2pyo, 2pz0.
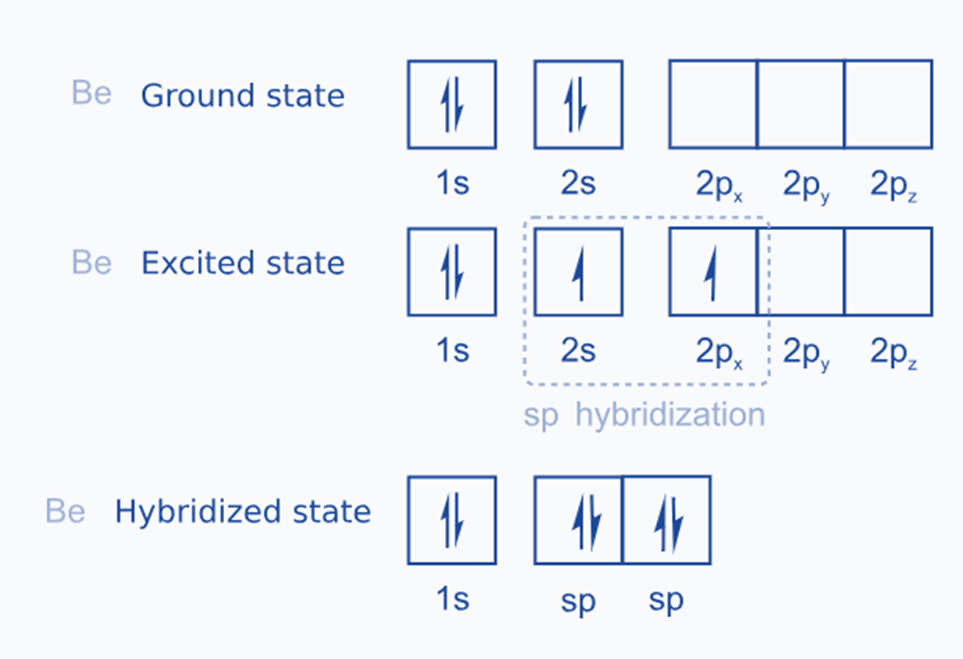
One 2s orbital and one 2p orbital of an excited beryllium atom are sp hybridized to generate two sp hybridized orbitals as in the figure below:
The two sp hybrid orbitals are linear and aligned at an angle of 180° in opposite directions. Two Be-Cl sigma bonds are formed when the sp-hybridized orbitals of each atom connect axially with the chlorine atom’s 3p half-filled orbital.
sp2 Hybridization
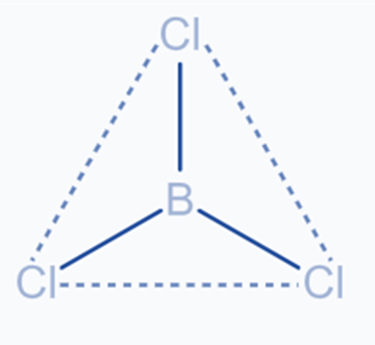
It occurs when the shells of one s and two p atoms combine to generate three equivalent orbitals. The newly produced orbitals are called SP2 hybrid orbitals. It is also known as trigonal hybridization. It includes combining one’s orbital with two ‘p’ orbitals of equal energy to form SP2, a new hybrid orbital. The trigonal symmetry of SP2 hybrid orbitals is retained at 120 degrees. All three hybrid orbitals stay in the same plane and make a 120° angle with one another.
Each hybrid orbital has a 33.33% and a 66.66% ‘p’ character.
The molecules with a triangular planar shape consist of a central atom, attached to three additional atoms, and are sp2 hybridized.
For example BCl3
The ground state configuration of a boron atom is 1S2, 2S2, 2Px1, 2Pyo, and 2Pz0.
Excited state configuration is 1S2, 2S1, 2Px1, 2Py1, , 2Pz0.
One boron 2s orbital combines with two excited boron atom 2p orbitals to generate three sp2 hybrid orbitals. Boron’s sp2 hybrid orbitals are planar and pointed toward the corners of an equilateral triangle. Each boron sp2 hybrid orbital overlaps axially with a chlorine atom’s 3p-half-filled orbital to generate three B-Cl sigma bonds.
sp3 Hybridization
In this hybridization, one orbital of the s sub-shell and three orbitals of the p sub-shell of the valence shell are mixed to generate four sp3 hybrid orbitals of equivalent energies and shape. Each sp3 hybrid orbital contains 25% s and 75% p characters.
These hybridized orbitals tend to be as far apart in space as feasible in order to minimize repulsive interaction between them. The four SP3 hybrid orbitals point to the four corners of a tetrahedron. The angle formed by the sp3 hybrid orbitals is 109.5°.
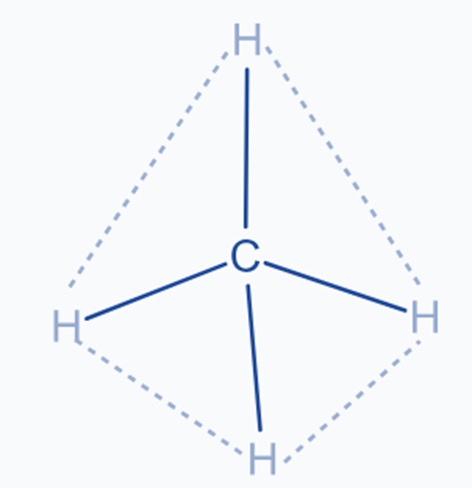
For example CH4
The carbon atom in the CH4 molecule undergoes sp3 hybridization. The central element, carbon, contains two unpaired electrons in p-orbitals in the ground state. One of the 2s electrons is promoted to the p orbital during excitation.
Ground state electronic configuration of C : 1S2, 2S2, 2Px1, 2Py1, 2Pz0
Excited state electronic configuration of C : 1S2, 2S1, 2Px1, 2Py1, 2Pz1
It now has four half-filled orbitals for bonding. By combining one 2S and three 2P orbitals, four SP3 hybrid orbitals are formed.
The carbon atom’s SP3 hybrid orbitals are pointed toward the comers of a normal tetrahedron. Each SP3 hybrid orbital overlaps axially with a half-filled 1S orbital of a hydrogen atom, forming a sigma bond.
Hybridization involving d orbitals
When d orbitals are either vacant or partially filled, they can participate in the hybridization process. Depending on the composition of the molecule, the s and p orbitals of the outermost shell can utilize the d orbitals of the outer shell as well as the d orbitals of the lower shell for hybridization. For example, 3d orbitals can hybridize with 3s, and 3p orbitals, as well as 4s and 4p orbitals. This is due to the fact that the energy of 3d orbitals is comparable to that of 3s and 3p orbitals, as well as the s and p orbitals of the 4th shell.
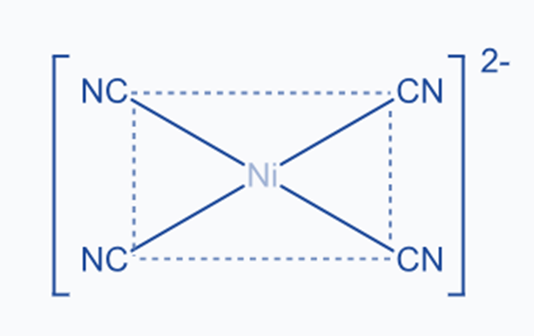
dsp2 Hybridization
It involves the intermixing of one ‘s’ orbital, two p orbitals, and one d orbital to generate four identical dsp2 hybrid orbitals. Each of the four hybrid orbitals is aligned to one of the four corners of a square. As a result of this, dsp2 hybridized molecules have square planar geometry. Because of the d-orbital, it is most commonly found in coordination compounds.
Nickel, for example, exhibits dsp2 hybridization in [Ni(CN)4]2-.
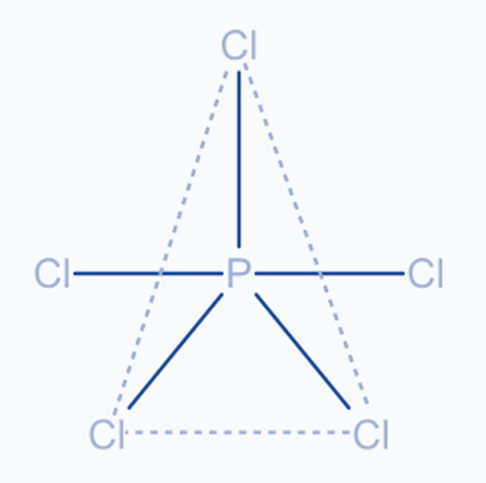
Sp3d hybridization
The combination of 1s, 3p, and 1d orbitals yields five sp3d hybridized orbitals of equal energy. They have a trigonal bipyramidal geometry. Trigonal bipyramidal symmetry is produced by combining the s, p, and d orbitals. The equatorial orbitals are three hybrid orbitals that reside in the horizontal plane and are arranged at a 120° angle to each other. The other two orbitals, known as axial orbitals, are in the vertical plane at 90 degrees to the equatorial orbitals.
For example, hybridization in Phosphorus Pentachloride (PCl5).
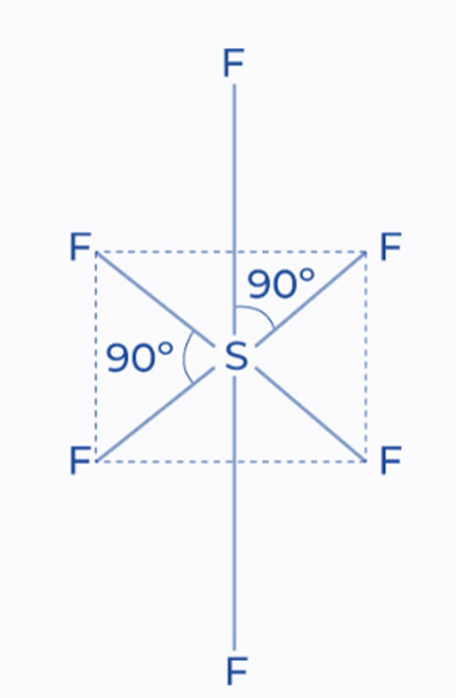
sp3d2 Hybridization
In this type of hybridization, six identical sp3 d2 hybrid orbitals are produced by mixing one s, three p, and two d orbitals together. These six orbitals are oriented toward the corners of an octahedron and intersect at a 90° angle in space. For example SF6
In a sulfur hexafluoride molecule, for example, sulfur is the central atom with two unpaired electrons in a 3p orbital. Six half-filled orbitals are required to form a bond with six fluorine atoms. It promotes one 3s electron and one 3p electron to two unoccupied d orbitals for this purpose. It possesses six unpaired electrons in an excited state. Six sp3d2 hybrid orbitals resulted from the hybridization of these orbitals.
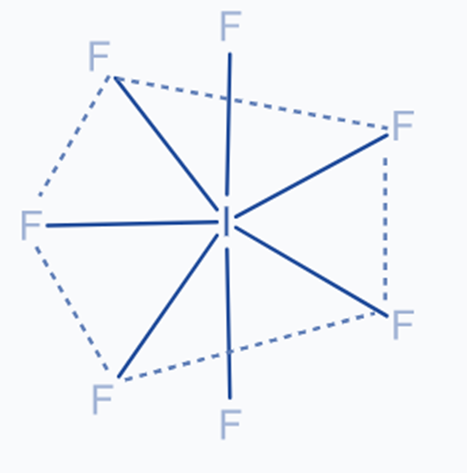
sp3d3 Hybridization
One s, three p, and three d atomic orbitals are combined together to form seven hybrid orbitals in this hybridization. Five of the hybrid orbitals have bond angles of 72o to the vertices of the pentagon, while the remaining two have bond angles of 90o to the plane. These orbitals are pointed toward the corner of the pentagonal bipyramidal. For example IF7
The central atom in iodine heptafluoride is iodine, which has one unpaired electron in a 5p orbital. It promotes one 5s electron and two 5p electrons to three unoccupied 5d orbitals, yielding seven excited unpaired electrons. These orbitals are combined to produce seven sp3d3 hybrid orbitals.
References
- Lee J. D. (1977). A new concise inorganic chemistry (3d ed.). Van Nostrand Reinhold. Retrieved July 6 2023 from https://archive.org/details/newconciseinorga00leej.
- https://ocw.mit.edu/courses/3-091-introduction-to-solid-state-chemistry-fall-2018/2382629922aac38ac001c282d96ad121_MIT3_091F18_REC10.pdf.
- https://uomustansiriyah.edu.iq/media/lectures/6/6_2021_09_19!01_45_44_PM.pdf.
- https://uomustansiriyah.edu.iq/media/le.ctures/6/6_2021_09_16!12_00_23_AM.pdf.
- https://byjus.com/jee/hybridization/.
- https://www.vedantu.com/chemistry/hybridization.

Thank you very much
Good
Nice