Hydrodynamic chromatography (HDC) is a technique used for separating particles based on their size, enabling the measurement of particle size distributions within the 20 – 1,000 nm range. This method expands the capability of traditional size exclusion chromatography to handle larger particles. Based on the distribution of particles in the flow streams of parabolic flow profiles that occur in tiny capillaries, the HDC principle operates. While larger particles are limited to the velocities at the capillary center, smaller particles can reach all flow streams. Larger particles thus elute ahead of smaller ones, in a manner akin to the separation order in size exclusion chromatography.
Interesting Science Videos
What is Hydrodynamic Chromatography?
- A liquid chromatographic method called hydrodynamic chromatography (HDC) separates analytes according to how big they are in the solution. Either an open tube or a column filled with inert, nonporous beads can be used for separation.
- The main objective of HDC is the quick separation of the liquid or solid components contained in the sample (also known as fractionation, or HDF, as another name for this process).
- Using preferential sampling of the streamlines of flow in the capillary or the interstitial medium of the packed column, sample components are separated in a size-dependent manner in HDC. The initial stage in characterizing size distributions and averages is this kind of division.
- The beads in a packed column HDC should be “inert,” meaning they should be composed of a material that reduces the enthalpic interactions between the beads and the dissolved analytes. To accomplish this “inertness,” surfactants and/or salts may need to be added to the solvent/mobile phase to screen out van der Waals and electrostatic interactions, particularly when working in aqueous media.
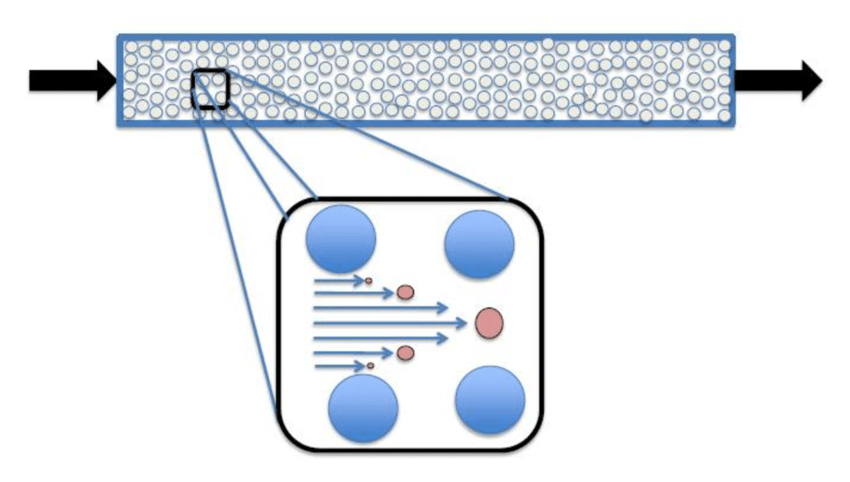
- The reason for separation is the parabolic or Poiseuille-like flow profile that forms in the open tube or interstitial medium of the packed column under laminar flow conditions. The fastest streamlines of flow are located in the center of the tube or interstitial medium, and the slowest streamlines are located close to the walls or packing particles.
- Larger analytes’ centers of mass in the sample will not be able to get as near to the packing particles or the tube walls as smaller analytes’ centers of mass can. Larger analytes will therefore stay close to the center of the flow profile, encountering the quicker streamlines more selectively and passing down the tube (packed column) at the average speed of these streamlines.
- In addition to experiencing the faster streamlines, the smaller analytes will also encounter the slower ones, resulting in an average velocity through the tube (packed column) that is slower than that of their bigger counterparts. Smaller analytes in the sample will elute from the tube (packed column) later than larger analytes as a result.
- Therefore, the elution order in HDC is the same as in size-exclusion chromatography (SEC), a method that is more widely used, with larger analytes eluting before smaller ones in both instances.
- Recent years have seen the resurgence of hydrodynamic chromatography (HDC) for the characterization of particles and polymers, primarily due to its association with numerous physical detection techniques.
- In a single study, HDC can identify the molar mass, size, shape, and structure of colloidal analytes continuously and as a function of one another when connected to light scattering (both multiangle static and quasi-elastic), viscometric, and refractometric detectors. Doing this exposes the analytes to less shear force than, say, size-exclusion chromatography, which reduces the possibility of flow-induced deterioration.
- Furthermore, the bioanalytical community has started to use microcapillary high-definition chromatography (HDC) more frequently. This technology has been used, among other things, to separate DNA fragments over a base pair range that spans four orders of magnitude.
In the fields of particle and polymer characterization, hydrodynamic chromatography appears to have finally matured, mostly due to the capability of multiple detection. With the latter, the analyte’s molar mass, size(s), shape, and structure can all be continuously and as functions of one another characterized. Even though the method has drawbacks such as poor chromatographic resolution, these can also be somewhat addressed by using the previously mentioned multi-detector approach. Technological improvements in the disciplines of macromolecular chemistry and particulate matter will undoubtedly influence the expanding field of bioanalytical chemistry, as will advancements in microcapillary HDC.
Principle of Hydrodynamic Chromatography
Hydrodynamic chromatography (HDC) operates on the principle of separating particles based on their size as they move through a flowing liquid within a chromatographic column. Unlike traditional chromatography methods where particles are separated based on their affinity for the stationary phase, HDC relies on the particles’ size-dependent diffusion rates within the flowing liquid. As particles of different sizes move through the column, smaller particles experience greater diffusion, leading them to elute later than larger particles. This process allows for the determination of particle size distributions within the analyzed sample.
How does Hydrodynamic Chromatography Operate?
- HDC operates similarly to Reld Sow Fractionation (FFF) and Size Exclusion Chromatography (SEC), except it only requires one (hydrodynamic) Reld and one (inert) mobile phase.
- The interesting idea behind particle separation is the variation in the rates at which particles are transported within a capillary-based on where in the eluent they are located.
- Larger particles elute more quickly than smaller ones because the former are preferentially located in the capillary’s center, where the flow rate is highest, while the latter is closer to the capillary wall, where the flow rate is nil.
- The separation factor (Rf) is the ratio of the maximum elution volume of a tiny molecule, known as a marker, to that of a particular particle.
- In addition to the process’s simplicity, the equipment’s ease of use and the speed with which measurements can be made immediately on the untreated medium are secondary benefits. Adjusting the operational parameters (sow rate, eluent, and additive type, capillary size, etc.) opens up a wide range of potential uses.
- There are two types of columns used for chromatography: packed and open capillary, where the channels are defined by the interstitial space. A high resolution can be achieved by using fine capillaries (diameter 4 μm) or very small particles (diameter 2 μm). For monodisperse polystyrene latexes, relatively short capillary columns (3 m) result in a quick and effective separation (5 min).
Hydrodynamic Chromatography for characterizing particle size, shape, and structure
Particle sizes and distributions were obtained by applying the kind of calibration curve, the majority of work on particle sizing via HDC used a single, concentration-sensitive detector (usually a UV detector, operated in so-called “lightobscuration” mode so that samples need not possess achromophore). The following detection techniques have been combined recently to create triple- and quadruple-detector HDC: differential viscometry (VISC), quasi-elastic light scattering (QELS, also known as dynamic light scattering), multi-angle static light scattering (MALS), and differential refractometry (DRI). This enables the analysis of the following:
- Using MALS + DRI, the molar mass distribution (MMD) and related statistical moments are obtained.
- The following size parameters’ distributions and corresponding statistical moments can be obtained:
- Radius of gyration (RG), using MALS + DRI
- Hydrodynamic (Stokes) radius (RH), using QELS +DRI
- viscometric radius (Rη), using MALS + VISC +DRI
- Sample shape and compactness as measured by the dimensionless parameter ρ≡RG,z/RH,z, which is determined by MALS + QELS.
- The sample’s structure and compactness are determined by the dimensionless ratio Rη,w/RG,z, which is derived by combining MALS + VISC + DRI.
Hydrodynamic Chromatography for characterizing ultra-high M polymers
- Ultra-high M polymers, which are often defined as polymers with M>1×106g/mol, have long been characterized using dual-detector HDC, but in recent years, the triple-detector technique (MALS + QELS + DRI or MALS +VISC + DRI) has been successfully used to this class of analytes as well.
- The polymers that travel through the packed, porous media of an SEC column acquire interstitial and through-pore stresses. As a result, very low flow rates must be used to prevent deterioration during SEC analysis, extending run times to several hours.
- Large polymers have been demonstrated to deteriorate in SEC even in these harsh circumstances, making precise characterization with this method difficult.
- This simpler approach can offer a way to describe ultra-high M polymers at a fraction of the time required to do so using SEC, or analyze polymers that cannot be accurately characterized by SEC since analytes only experience interstitial stresses rather than pore stresses in HDC.
- Though HDC with low-angle static light scattering and UV detection was applied 25 years ago to the study of polyacrylamides, and HDC/MALS/UV has been used to study the coil-stretch transitions of synthetic polymers and the transition from HDC to a slalom chromatography mode of separation within the columns, the majority of multi-detector HDC applications in the polymeric arena have been to polysaccharides such as alternan and to the starch components amylose and amylopectin.
Microcapillary Hydrodynamic Chromatography for Characterizing DNA Fragments
- Due to the challenge of using the technique on non-polystyrene latexes, single-detector (primarily UV) capillary HDC commercial instruments did not find much acceptance in the particle sizing field; however, microcapillary HDC has seen success in recent years for the analysis of calculations.
- The microcapillary HDC method was used to separate DNA fragments ranging in base pair count from 75 to 106,000.
Application of Hydrodynamic Chromatography
- Nanoparticle Characterization: HDC is widely used in the analysis of nanoparticles, allowing for precise determination of particle size distributions in nanometer ranges. This is crucial in various industries such as pharmaceuticals, cosmetics, and materials science.
- Polymer Analysis: The molecular weight distributions of polymers can be measured and their characteristics can be characterized with HDC. It supports polymer chemistry research and quality control by offering insights into the size and structure of polymer chains.
- Environmental Monitoring: In environmental studies, HDC can be employed to analyze particulate matter in air, water, and soil samples. It helps in understanding the distribution of pollutants, microplastics, and other particles, contributing to environmental monitoring efforts.
- Biomedical Research: HDC is useful in the study of biological nanoparticles, including viruses, liposomes, and protein aggregates, in biomedical research. It makes it easier to analyze biomarkers, drug delivery methods, and interactions between biomolecules.
- Food and Beverage Industry: In the food and beverage industry, HDC is used for analyzing particle size distributions in various products, including emulsions, suspensions, and colloidal systems. It assists in optimizing product formulations and ensuring product stability.
- Cosmetics Formulation: By describing the size distribution of the particles in creams, lotions, and emulsions, HDC contributes to the formulation of cosmetic products. It facilitates the achievement of the intended performance, texture, and appearance of cosmetic compositions.
- Pharmaceutical Development: HDC is used in the pharmaceutical industry to analyze pharmaceutical excipients, drug formulations, and nanoparticles for drug delivery. It helps to maximize the pharmacokinetic, bioavailability, and stability of drugs.
- Quality Control: HDC serves as a valuable tool for quality control in various industries, allowing for the rapid and accurate analysis of particle size distributions. It helps in ensuring product consistency, identifying contaminants, and troubleshooting manufacturing processes.
Uses of Hydrodynamic Chromatography in Polymer Chemistry
- Colloids: The heterogeneous process of free-radical emulsion polymerization yields these particles. Their diameter is approximately 100 nm, which HDC can monitor.
- Polymerization kinetics: One of the main applications of HDC is in colloids produced by emulsion polymerization, which permits high molecular weights and rapid polymerization rates to occur simultaneously. The HDC measurement’s speed works well with kinetic research and polymerization monitoring.
- Modification of Latexes: The Ugelstad approach has proven to be effective in producing large-size latex by further polymerizing the colloids generated in the first step. Depending on their stability, latexes exhibit varying degrees of reactivity. In comparison to other techniques, HDC has found flocculation of colloids in the presence of inorganic oxides or water-soluble ionic polymers. The interaction between particles and thickeners has been amply shown; yet, aggregates and intense shear can disrupt this association.
Advantages of Hydrodynamic Chromatography
- High Resolution: Hydrodynamic chromatography, particularly in the nanoscale range, provides high resolution for size-based particle separation. This makes it possible to accurately characterize complicated samples with a broad range of sizes.
- Broad Range of Applications: HDC is adaptable and useful in several industries, such as biotechnology, pharmaceuticals, polymers, nanotechnology, and environmental science. It is capable of analyzing a wide variety of particles, such as biological particles, proteins, polymers, and nanoparticles.
- Minimal Sample Preparation: HDC usually necessitates less sample preparation than certain other chromatographic procedures. This makes the analytical process simpler and lowers the possibility of changing the sample’s attributes.
- Non-Destructive Analysis: HDC is a non-destructive technique, which means that following analysis, the examined samples are left undamaged. For applications later on or for additional characterization, this is beneficial.
- Quantitative Analysis: Particle size distributions can be quantitatively analyzed using hydrodynamic chromatography. Particle concentrations can be accurately quantified by coupling with suitable detectors, such as UV-vis or multi-angle light scattering detectors.
Disadvantages of Hydrodynamic Chromatography
- Restricted Particle Size Variety: HDC may not be able to handle particles at the extremities of the size spectrum, even though it can manage a large variety of sizes. Particles that are either exceedingly small or extremely huge could make separation and detection difficult.
- Complex Instrumentation: High-dynamic-pressure systems (HDC) can be intricate and need specific instruments, such as accurate pumps, detectors, and columns. Higher initial setup expenses and maintenance requirements may follow from this.
- Long Analysis Time: HDC analysis can take a while, depending on the sample complexity and column characteristics. This might make it less useful in situations requiring quick results or large throughput.
- Sensitivity to Sample Composition: In complicated mixes, in particular, the sample matrix composition can have an impact on the HDC’s performance. The efficiency and repeatability of separation can be impacted by interactions between the materials of the column and sample components.
- Problems with Data Interpretation: It can be difficult to analyze HDC data, particularly for complicated samples that have overlapping peaks or wide-size distributions. Accurate outcomes require proficiency with data interpretation and calibration.
References
- Derek Lohmann, “Hydrodynamic Chromatography an efficient to tool to quickly determine particle size distributions in the sub-micron range”
- André M. Striegel, “Hydrodynamic chromatography: packed columns, multiple detectors, and microcapillaries”, Anal Bioanal Chem (2012) 402:77–81(DOI 10.1007/s00216-011-5334-3).
- A. Revillon, “Hydrodynamic Chromatography: Practical Applications”.
- A. J. McHugh, “Hydrodynamic chromatography”.
- André M. Striegel and Amandaa K. Brewer, “Hydrodynamic Chromatography”, Annual Review of Analytical Chemistry” (https://doi.org/10.1146/annurev-anchem-062011-143107).
