Inorganic polymers are polymers whose backbone structure is made up of elements other than carbon.
They are polymers with inorganic repeating units in their main polymeric structure. They have distinctive Physico-chemical characteristics and unique physical, mechanical and electrical properties. Their applications include contact lenses and medical devices, as well as shampoos, food (antifoaming agents), caulking, and lubricating oils. Polydimethylsiloxane, glass, and polyphosphates are the best-known examples of inorganic polymers.
Interesting Science Videos
Characteristics of inorganic polymer
- Inorganic polymers are more strong, more hard, and brittle than organic polymers.
- At high temperatures, they soften, i.e., melt but do not burn (except for polymers containing sulfur).
- They have a high density of covalent bonds.
- In nature, they are present as pure amorphous or pure crystalline.
- They have low-temperature flexibility.
The significance of inorganic polymers over organic polymers
Most organic polymers burn, liberating toxic smoke in the process, and many degrade when exposed to ultraviolet or gamma radiation. Organic polymers can soften at low temperatures or dissolve in organic solvents, oils, or hydraulic fluids.
As the valencies of inorganic elements differ from those of carbon, the number of side groups attached to a backbone may differ from that of an organic polymer. This can have an impact on the macromolecule’s flexibility. Bonds formed by inorganic elements are frequently longer, stronger, and more resistant to free radical cleavage reactions than carbon bonds.
Classification of polymers based on composition
Whether the polymer contains atoms of only one element or different elements in its backbone, they are classified into the following two groups.
Homopolymer
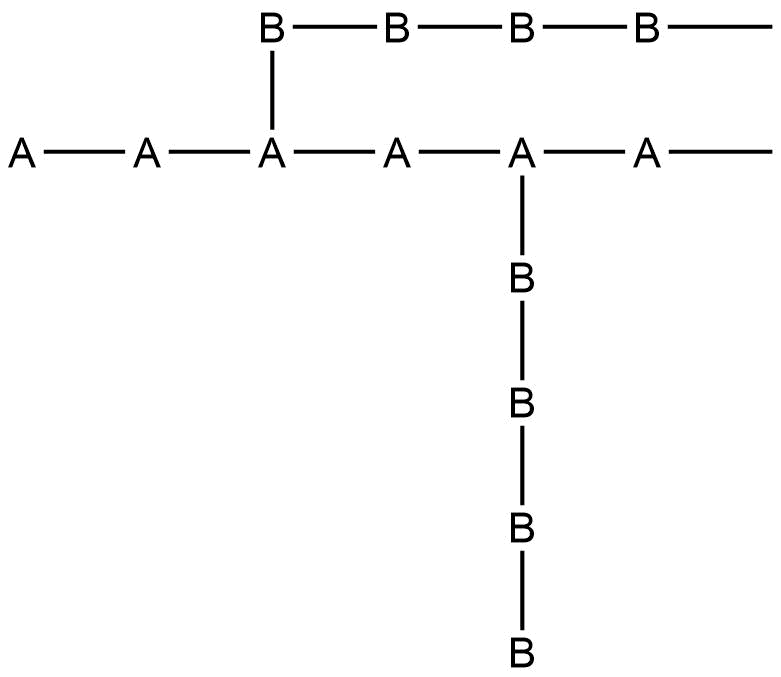
The polymers which contain identical monomers are homopolymers. The catenation forms them. Silicon, Sulphur, germanium, and tin form homopolymers. Generally, they are linear and long-chain in structure and are thermally less stable. They consist of identical repeating units, which the formula can represent, -A – A – A – A -A – A-.
Heteropolymer
These polymers have atoms of various elements in their backbones. They are formed by alternating two or more different atoms in their backbone structure. Quartz, phosphazine, tetrasulphur, and boron nitride are examples of heteropolymers.
Copolymer
Copolymers are polymers that consist of two or more monomer species. The majority of commercial polymers contain two kinds of monomers, but some of these contain three molecules, also called terpolymers.
There are four types of copolymer:
i. Alternating copolymer: In alternating copolymers, monomers or repeat units are present in order sequence. There is an alternate distribution of repeat units throughout the polymer chain. Monomer units are in regular session.
– A – B – A – B – A – B – A -B –
ii Random copolymers: In this polymer, monomer subunits are in random order. Monomers with similar bond polarities are more likely to form a random copolymer. The distribution of monomers unit along the chain is random.
– A – A – B – A – B – B – A – A -A.
iii Block copolymer: When a sequence or block of one repeat unit follows one another along the main chain polymer chain. There is the presence of a linear arrangement of blocks of the molecules.
-A – A – A – B – B – B – A – A – A – B – B – B –
iv: Graft copolymer: Graft copolymers are polymers containing two or more different chemical chains, with multiple branches developed from polymeric chains. The branch chains have different chemical compositions than the backbone chain. The side chains of different repeat units have been grafted on a linear polymer chain. Thus the name is graft polymer.
Classification based on connectivities
N. H. Ray uses connectivity as a method of classifying inorganic polymers in his book on inorganic polymers. He defines connectivity as the number of atoms attached to a specific atom that is part of a polymer. The connectivity of a polymer can vary from one for a side group atom or functional group to at least 8 or 10 in some metal coordination and metal cyclopentadienyl polymers.
Connectivities of 1
Anchored metal-containing polymers used for catalysis can have connectivity values as low as 1 concerning the other simple polymer chain, as in the figure below:
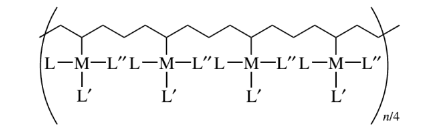
Other ligands can be present in the metal, but as long as they do not affect the polymer connectivity, the metal is defined as having a connectivity of 1.
Connectivities of 2
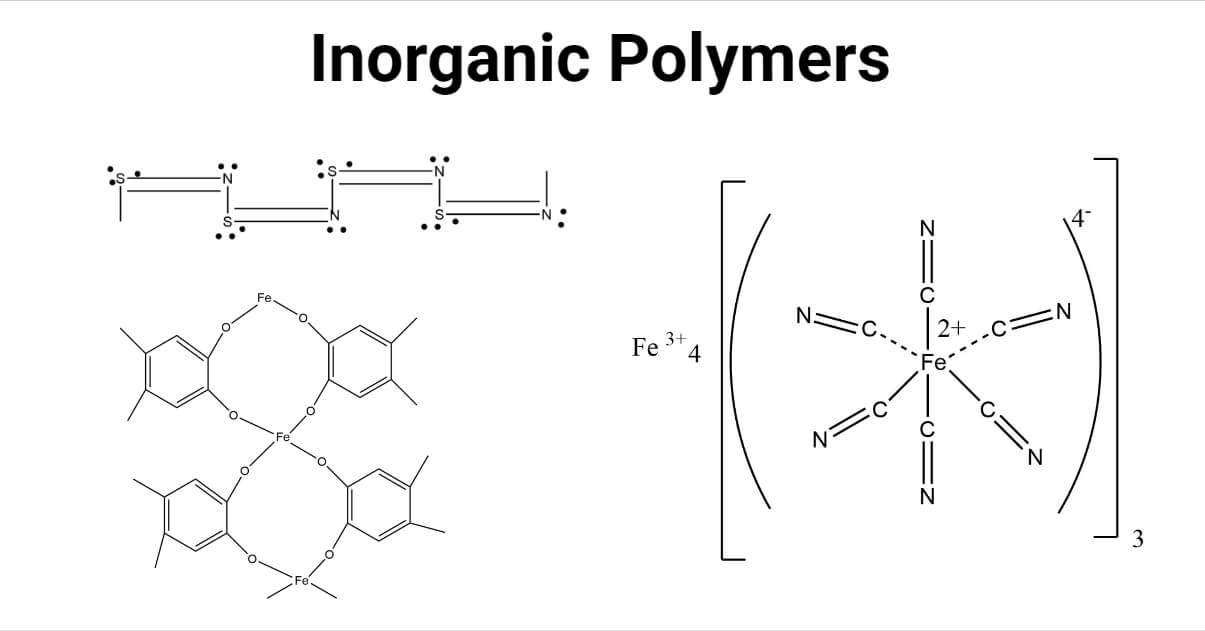
Sulfur and selenium have connectivity of 2 in their chain polymer allotropes. In addition, they have connectivity 2 in their ring structure.
Connectivities of 3
Boron in boric oxide, silicon in silicates such as mica, talc, and pyrophyllite, and carbon in graphite have connectivities of 3. Such connectivities provide two-dimensional polymers that are film and sheet-forming materials and are good lubricants.
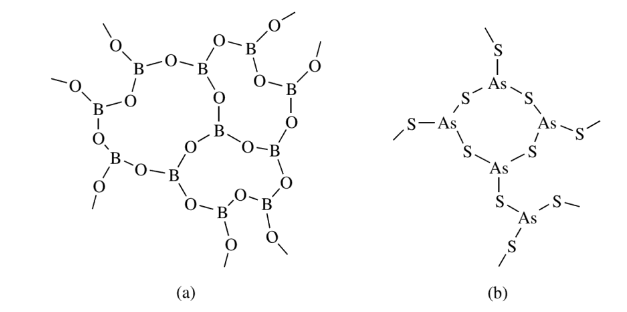
Connectivities of 4
The silicon atoms in vitreous silica have a connectivity of 4. Boron and aluminum phosphates, as well as many other three-dimensional polymers, have connectivities of 4 for at least one type of atom.
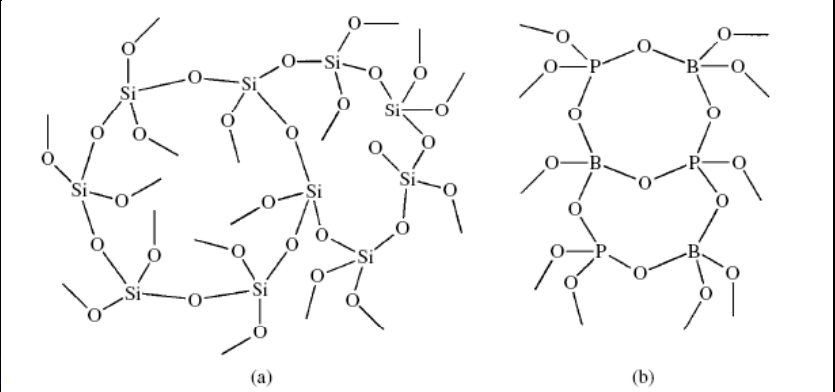
Mixed connectivities of 3 and 4
Inorganic polymeric species like borate glasses have mixed connectivities of 3 and 4. The counter cations provide the counter charge for the four oxide ions connected to some of the boron atoms.
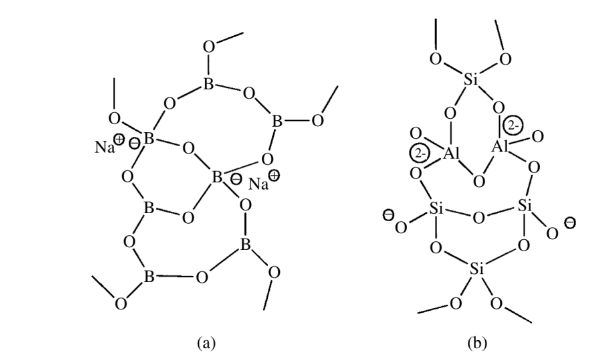
Connectivity of 6
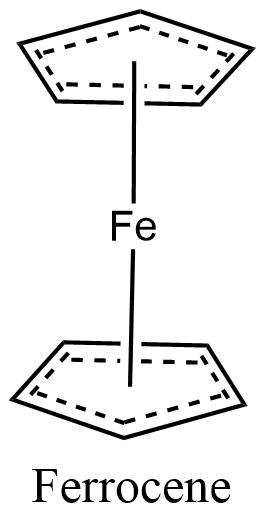
Metal coordination polymers with metal atoms or ions linked with two tridentate ligands are considered to have the connectivities of 6. If the cyclopentadienyl ring is considered connectivity of three, then the ferrocene polymers can be considered to have iron atoms with connectivities of 6.
Mixed connectivities of 4 and 6
Titanium, zirconium, tin, cerium, thorium, silicon, germanium orthophosphates and arsenates have mixed connectivities of 4 and 6. The figure shows an example.
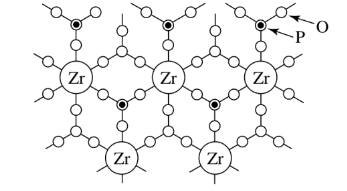
Connectivities of 8
Metal coordination polymers of zirconium (IV), yttrium (III), and several lanthanide ions [cerium (IV), lanthanum (III), europium (III), gadolinium (III), and lutetium (III)] with connectivities of 8 have been synthesized they consist of two tetradentate ligands coordinated to each metal ion that is part of the polymer chain.
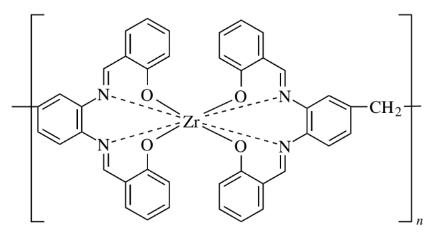
Classification of inorganic polymers based on dimensionality
There are three types of inorganic polymers based on dimensionality. Pittman et al. use this classification to describe polymeric species with metal ions in their backbones.
1-D polymeric structure
Linear chain polymers are categorized as one-dimensional polymers even though they have twist and turn in the linear chain.
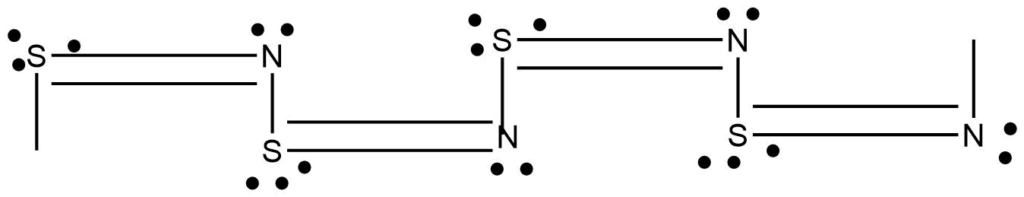
2-D polymers
The polymers with connectivity three generally form sheet or two-dimensional polymers. E.g., sulfide and graphite. For the 2-d dimensional polymer, at least one type of atom must have connectivity of three or more.
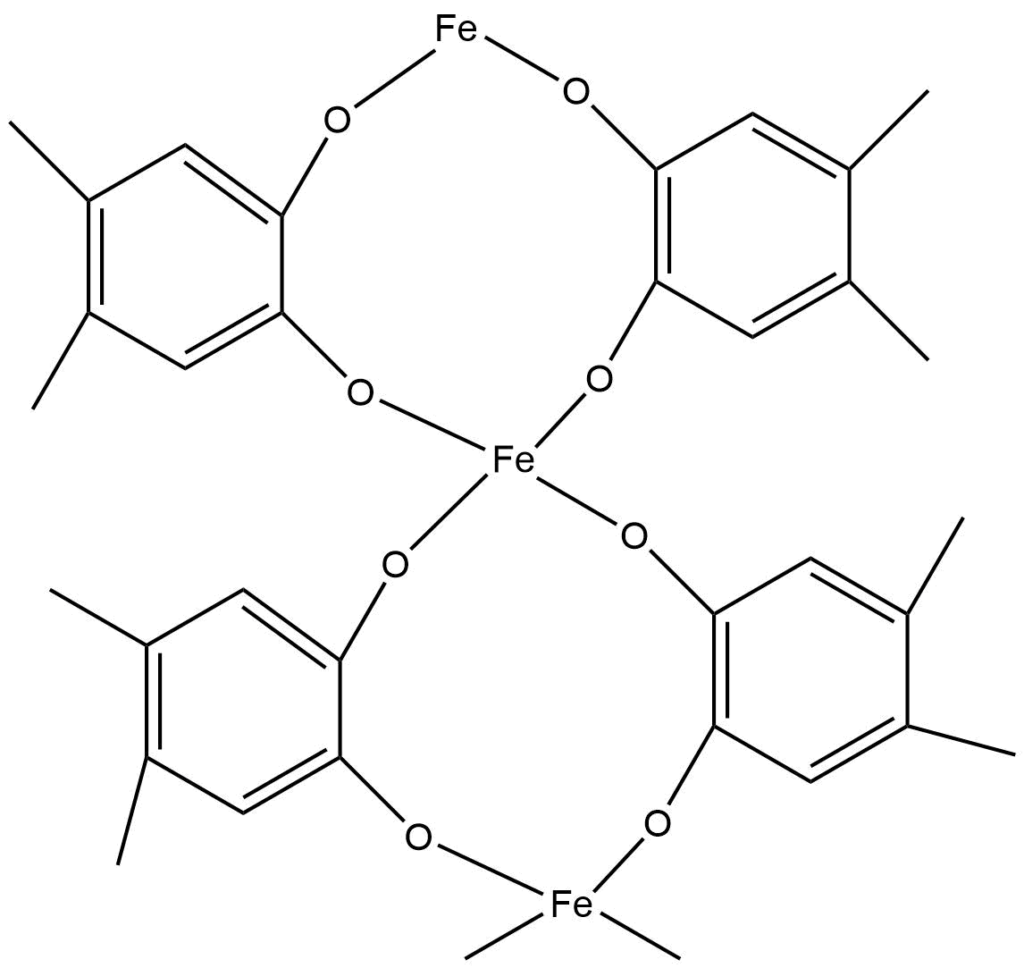
3-D polymers
There are various inorganic polymeric networks with three-dimensional bonding. For a true 3-D polymer, at least some of the atoms must have connectivity of 4 or higher. Prussian blue is a typical example of a mixed Fe (II) and Fe (III) 3 – D polymeric structure, with each iron ion surrounded by six cyano ligands octahedrally.
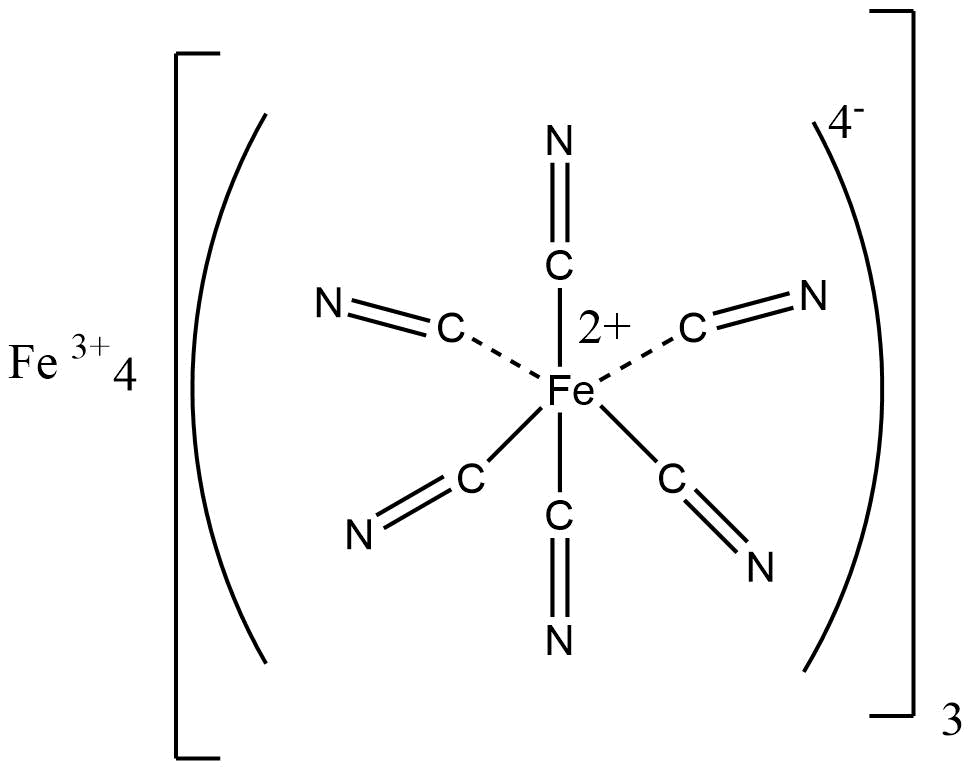
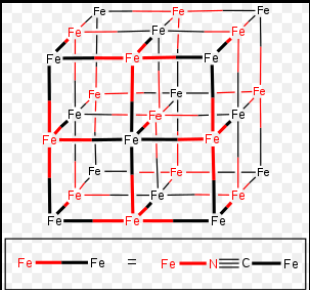
source: https://en.wikipedia.org/wiki/Prussian_blue
Classification of inorganic polymer based on the chemical composition
There are four different types of inorganic polymers based on their chemical composition. They are as follows:
Wholly inorganic polymers
Inorganic polymers of this class include major components of soil, mountains, and sand. They have a wide range of applications as abrasives and cutting materials. Polymers in this class include carbon black, silica gel, aluminium silicate, clays, lubricants, and catalysts (zinc oxide, nickel oxide).
Inorganic – organic polymers
These inorganic polymers consist of organic portions attached to inorganic elements in their backbone. The field of inorganic-organic polymers is very broad. Polysilanes, polysiloxanes, and polyphosphazene are some examples of this class.
Organometallic polymers
They consist of compounds with a covalent bond between a carbon atom and a metal. Organometallic polymers are made of over 40 elements, including the main group of metals (si or ge), transition metals, or rare earth elements, in addition to the elements C, H, N, O, B, and P found in organic polymers.
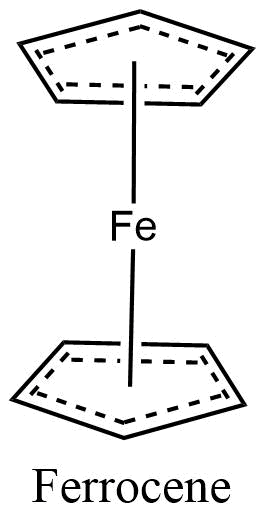
Organometallic polymers are novel materials that combine the low density, structural diversity, and functional group varieties of organic materials with the electrical conductivity and high-temperature stability properties of inorganic compounds.
Classification of inorganic polymers based on the types of reaction
Addition (polymerization) polymers
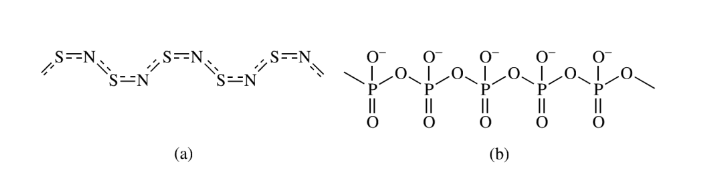
The combination of simple molecules to form a single giant molecule without the elimination of any small molecules gives the addition of polymers. The process is called addition polymerization. Polyphosphonitric chloride and sulfur nitride are examples of inorganic addition polymers.
Condensation polymers
The condensation polymerization process involves the condensation or combination of two or more polyfunctional molecules to form large macromolecules and eliminate small molecules like H2O, NH3, H2, and HCl. The polymers formed by this process are condensation polymers. Polyamides, silicons, borazine, and polysiloxanes are inorganic condensation polymers.
Co-ordination polymers
Polymers formed by combining ligands with metal ions are called coordination polymers. These polymers contain chelated metal atoms and ions. Dimers of AlCl3 are an example of a coordination polymer.
Applications of inorganic polymers
- They are used as greases and lubricants.
- Waterproofing electrical condensers also consist of inorganic polymers.
- They have wide applications in ceramics, rubber, and plastics.
- Medical prosthetic applications of the polymer include heart valves, stents, cartilage scaffolds, joints, and the creation of artificial skin.
- Sensors and biosensors consist of inorganic nanocomposites of metal and metal oxides. They have wide environmental and medical applications.
- Phosphoesters are used in medicine as nerve conduits and drug and gene delivery matrices.
- Petrochemical industries also use inorganic polymers.
References
- https://onlinelibrary.wiley.com/doi/10.1002/0471224456.ch1
- https://theskyofchemistry.blogspot.com/2020/07/classification-of-inorganic-polymers.html
- http://www.lscollege.ac.in/sites/default/files/e-content/classificationofinorganicpolymers-priyanka.pdf
- https://pubs.rsc.org/en/content/articlelanding/1969/rr/rr9690200013
- https://books.google.com.np/bookshl=en&lr=&id=9KFwwpv6bGcC&oi=fnd&pg=PR7&dq=Inorganic+and+Organometallic+Polymers+Ronald+D.+Archer&ots=jRI7e4BuQl&sig=YqYoLguRmBhb8PGH2oElwiFP00M&redir_esc=y#v=onepage&q&f=false
- datenpdf.com_mpk-introduction-to-inorganic-polymer
- https://chem.libretexts.org/Ancillary_Materials/Worksheets/Worksheets%3A_Inorganic_Chemistry/Worksheets%3A_Structure_and_Reactivity_in_Organic_Biological_and_Inorganic_Chemistry/251_Workbook/27%3A_Inorganic_polymers

It’s so nice