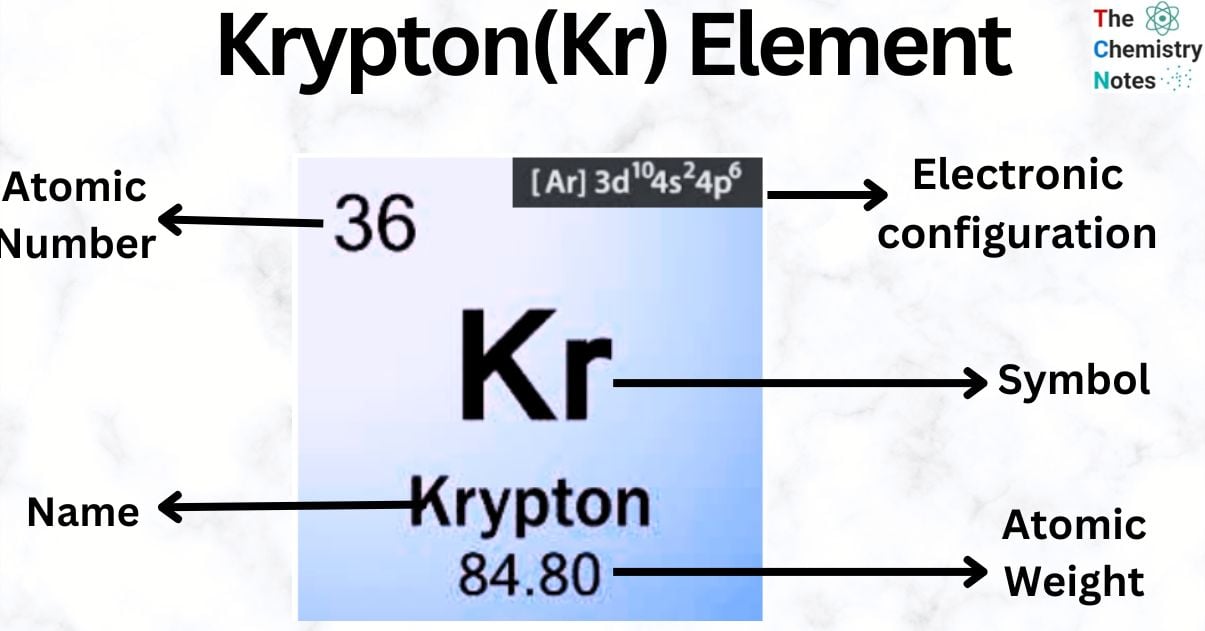
Krypton is a chemical element with the atomic number 36 and is represented by the symbol ‘Kr’ in the periodic table. It belongs to the p-block of group 18 of the periodic table. It is a rare gas that is three times heavier than air. It is colorless, odorless, and tasteless. The earth’s atmosphere contains approximately 0.0001% krypton.
Interesting Science Videos
History of Krypton
- Sir William Ramsay, a Scottish chemist, and Morris M. Travers, an English chemist, discovered Krypton on May 30, 1898.
- They did this by evaporating the liquid gas. Small amounts of liquid krypton remained after the more volatile components of liquid air had boiled away.
- After a few weeks, he discovered a new gas, neon, by employing a similar method.
- In 1904, William Ramsay was awarded the Nobel Prize in Chemistry in recognition of his services in the discovery of inert gaseous elements in the air and his determination of their position in the periodic system. He was responsible for adding a completely new group to the periodic table. The only noble gas he did not discover was radon.
- The name of the element derives from the greek word “kryptos” which means hidden.
Occurrence of Krypton
- Krypton is a gas that is rare in the atmosphere of Earth. The earth’s atmosphere contains approximately 0.0001% krypton.
- The disintegration of uranium or other radioactive elements may result in extremely minute amounts of krypton in the Earth’s crust.
- Krypton can be recovered by fractionally distilling liquefied air and extracting carbon dioxide, nitrogen, water vapor, and oxygen from the remaining liquefied air residues.
- Krypton’s abundance in space is unknown because it is estimated by meteoric activity and solar winds. The initial findings indicate that krypton is abundant in space.
Isotopes Of Krypton
Krypton has six naturally stable isotopes,which include; 78Kr, 80Kr, 82Kr, 83Kr, 84Kr,and 86Kr
Naturally occurring Krypton isotopes
| Isotopes | Natural abundance (atom %) |
|---|---|
| 78Kr | 0.35 (1) |
| 80Kr | 2.28 (6) |
| 82Kr | 11.58 (14) |
| 83Kr | 11.49 (6) |
| 84Kr | 57.00 (4) |
| 86Kr | 17.30 (22) |
Elemental Properties of Krypton
| Electronic Configuration | [Ar] 3d104s24p6 |
| Atomic Number | 36 |
| Atomic Weight | 83.798 g.mol -1 |
| State at 20°C | Gas |
| Group, Period, and Block | 18, 4, p-block |
| Density | 0.003425 g.cm -3 at 20 °C |
| Van der Waals radius | 0.197 nm |
| Electron shells | 2, 8, 18, 8 |
| Electrons | 36 |
| Protons | 36 |
| Neutrons in most abundant isotope | 48 |
Physical Properties of Krypton
- Krypton is an element in a gaseous state. It has a melting point of −157.37°C (−251.27°F ) and a boiling point of −153.415°C (−244.147°F).
- The density of Krypton is 0.0037 grams per cubic centimeter.
- Krypton is diamagnetic (i.e., it is repelled by an external magnetic field).
- Krypton gas weighs nearly three times as much as air.
- Krypton is white and crystalline in solid form.
- It is odorless,colorless,and tasteless.
| Color/physical appearance | Colorless |
| Melting point/freezing point | −157.37°C, −251.27°F, 115.78 K |
| Boiling point | −153.415°C, −244.147°F, 119.735 K |
| Density | 0.003425 g cm-3 at 20°C |
| Electronegativity | 3.0 [Pauling Scale] |
Chemical Properties of Krypton
- Krypton has six naturally occurring stable isotopes.
- The electronegativity of krypton on the Pauling scale is 3.00.
- Krypton has two oxidation states.
- Krypton is unreactive so it is also known as inert or noble gas.
Chemical Reaction Of Krypton
- Reaction of Krypton with Water
Krypton does not react with water. It does, however, dissolve slightly to the extent of about 59.4 cm3 kg-1 at 20°C (293 K).
- Reaction of Krypton with Air
Krypton does not react with an air.
- Reaction of Krypton with the Halogens
When a mixture of krypton and fluorine, F2, is cooled to -196°C and zapped with an electric discharge or X-rays, the result is the formation of the difluoride krypton (II) fluoride, KrF2. This compound decomposes on warming to room temperature. The other halogens do not react with krypton.
Kr (s) + F2 (s) → KrF2 (s)
Krypton does not react with acid and bases.
Uses Of Krypton
Because of the rarity of the gas, krypton is difficult to obtain, which makes its application difficult. However, there are still many uses of krypton, which include:
Used As Insulator: Krypton is also known for better insulation among window panels than argon, allowing for more energy-efficient windows. Krypton is ideal for large window frames, particularly large commercial windows. In fact, argon cannot be utilized if the distance between glass panels is less than half an inch, in which case krypton must be used instead.
Used In Strong Light: It is utilized in the production of white lighting bulbs for cinematography. It’s been utilized to make camera flashes for high-speed photography. Krypton, when combined with Mercury, creates a dazzling light that has been utilized to create the illuminating sign that directs airplanes on the runway during foggy weather. It is also employed in the production of energy-efficient fluorescent lamps.
Used In Electronics: Krypton is an important component of neon-based excimer laser gas mixtures, which are employed to generate laser light for precise photolithography in semiconductor production.
Used In Glass Manufacturing: Krypton is used to make specialty flat glass for the solar, automobile, and construction industries. The application of krypton during the coating procedure allows the glass to be created with unique and specialized properties.
Health Effects Of Krypton
- This gas is classified as a basic asphyxiant and is inert.
- Krypton-85 is extremely toxic and has been linked to cancer, thyroid disease, skin, liver, and renal problems.
- Prolonged inhalation can cause dizziness, nausea, vomiting, loss of consciousness, and death. Death can occur as a result of poor judgment, confusion, or loss of consciousness, which prevents self-rescue. When oxygen levels are low, unconsciousness and death can ensue in seconds.
Environmental Effect Of Krypton
Because krypton is a rare atmospheric gas, it is non-toxic and chemically inert. The extraordinarily freezing temperature (-244 °C) will instantly freeze species, but no long-term ecological repercussions are expected.
Handling and Storage of Krypton
- Cylinders containing krypton can be stored both indoors and outdoors, however the latter must be shielded from atmospheric precipitation and sunshine.
- Cylinders containing krypton may be stored in open areas alongside other air separation products, provided that the storage areas are separated by non-combustible barriers 1.5 m high. J
- oint storage with cylinders containing combustible gases is also permitted, provided that the storage areas are separated by non-combustible protective walls 2.5 m high.
- Cylinder storage should be done with the caps screwed on.
- At 20 °C, the nominal pressure of krypton should not be less than 9.8 MPa (100 kgf/cm2).
- When in use, the gas in the cylinders must not be totally consumed. The cylinder’s residual gas pressure must be at least 0.05 MPa (0.5 kgf/cm2).
Facts about Krypton
- In our cosmos, Krypton is assessed by solar winds and meteoric activity. Although scientists are unknown how much krypton there is in our cosmos,
- Krypton-81 can be used to measure the age of ice in Antarctica. They compare the amount of krypton-81 in ice bubbles to the amount of krypton-81 in our environment now. They can establish the age of the ice based on the rate of degradation.
- Superman is the most well-known fictional superhero who used kryptonite to combat bad powers. Kryptonite is said to be capable of separating Superman from his powers, although this is simply a supposition because no such material has ever been discovered on Earth. Don’t get krypton mixed up with kryptonite, the famous Superman repellant. Kryptonite is a radioactive material that ranges in hue from red to green to black.
Watch out for the video to have a recap of all the information related to Krypton.
References
- Bartlett, Neil (2003). “The Noble Gases”. Chemical & Engineering News. Retrieved 2006-07-02.
- William Ramsay, The Recently Discovered Gases and Their Relation to the Periodic Law., Science, 1898, Vol. IX, p273-280.
- https://chemistrytalk.org/krypton-element/
- https://www.rsc.org/periodic-table/element/36/krypton
- Leonello Paoloni, The noble gas compounds: the views of William Ramsay and Giuseppe Oddo in 1902., J. Chem. Educ., 1983, 60 (9), p758.
- D. R. MacKenzie, Krypton Difluoride: Preparation and Handling., Science 20 September 1963, Vol. 141 no. 3586 p1171.
- https://byjus.com/chemistry/krypton/

