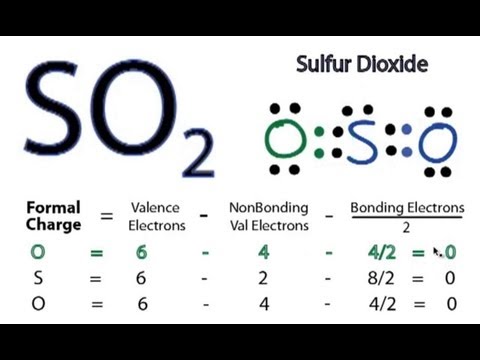
Drawing the Lewis structure of SO2 is critical for understanding its molecular bonding and chemical characteristics. The Lewis structure predicts the molecular shape, polarity, and reactivity. With practice, you will become skilled at drawing Lewis structures and have a deeper understanding of molecular bonding.
Interesting Science Videos
Lewis Structure of SO2
A sulfur atom (S) and two oxygen atoms (O) make up the SO2 Lewis structure. The sulfur atom (S) is the center atom, and the two oxygen atoms (O) surround it at a bond angle of 119 degrees. The sulfur atom (S) and each oxygen atom (O) form two double bonds. The two oxygen atoms (O) each have two lone pairs, while the sulfur atom (S) has one.
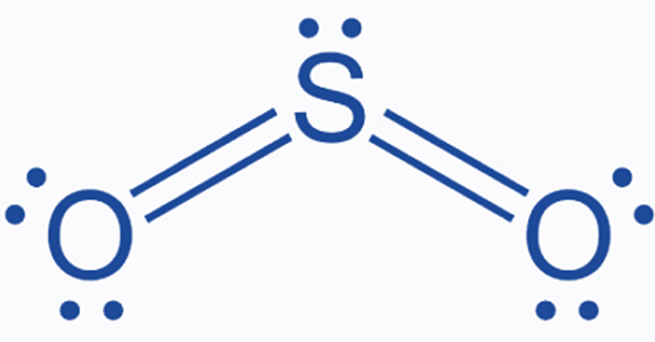
How To Draw Lewis Structure of SO2
- Determine the total valence electrons.
Begin by determining the valence electrons of each atom in the molecule. Sulfur in SO2 is in Group 6, which means it contains 6 valence electrons, while each oxygen atom in Group 6 provides 6 electrons.
As a result, the total number of valence electrons in SO2 is 6 + 2(6) = 18.
- Determine the central atom
Keep in mind that the atom with the least amount of electronegative stays in the center when choosing the center atom. Now, the provided molecule is SO2 (sulfur dioxide), which comprises both sulfur (S) and oxygen atoms.
The sulfur atom is less electronegative when we compare the electronegativity values of sulfur (S) and oxygen (O).
In this particular case, the oxygen (O) atoms are the outer atoms and the sulfur (S) atoms are the central atoms.

- Put a pair of electrons between each atom to form a connection
The sulfur (S) and oxygen (O) atoms in the SO2 molecule now need to have electron pairs positioned between them.

- Make the surrounding atoms stable.
Position the remaining valence electron pair on the center atom. This stage requires you to check the stability of the outer atoms. The outside atoms in the drawing of the SO2 molecule are oxygen atoms. These outer oxygen atoms form an octet, and so are stable.

Also, in step 1, we calculated the total number of valence electrons in the SO2 molecule.
The SO2 molecule possesses a total of 18 valence electrons, but only 16 are used in the above illustration.
So the number of electrons left is 18 – 16 = 2.
These two electrons must be placed on the central sulfur atom in the SO2 molecule.

- Check the octet on the center atom.
If it does not have an octet, shift the lone pair to establish a double or triple bond.
In this phase, determine whether the central sulfur atom (S) is stable or not.
To assess the stability of the central sulfur (S) atom, we must first determine if it is forming an octet.
Unfortunately, the sulfur atom does not form an octet here. Sulfur has only six electrons and is unstable. To make this sulfur atom stable, you must move the electron pair from the outer oxygen atom, allowing the sulfur atom to have 8 electrons.

The sulfur atom, with 8 electrons, forms an octet.

Let us now move on to the final phase, which is to determine whether the Lewis structure of SO2 is stable.
- Check the stability of the Lewis structure.
The concept of formal charge can be used to verify the stability of a Lewis structure.
In summary, you must now determine the formal charge of both the sulfur (S) and oxygen (O) atoms in the SO2 molecule.
To calculate the formal charge, use the following formula:
Formal charge = Valence electrons – (Bonding electrons)/2 – Nonbonding electrons
For Sulfur (S) atom:
Valence electrons = 6 (because sulfur is in group 16)
Bonding electrons = 6
Nonbonding electrons = 2
Formal charge = 6 – 2 – 6/2 = 1
For double bonded Oxygen (O) atom:
Valence electrons = 6 (because oxygen is in group 16)
Bonding electrons = 4
Nonbonding electrons = 4
Formal charge = 6 – 4 – 4/2 = 0
For single bonded Oxygen (O) atom:
Valence electrons = 6 (because oxygen is in group 16)
Bonding electrons = 2
Nonbonding electrons = 6
Formal charge = 6 – 6 – 2/2 = -1
According to the aforementioned calculations of formal charge, the sulfur (S) atom has +1 charge and the single bonded oxygen (O) atom has -1 charge.
As a result, the previously determined Lewis structure of SO2 is not stable.
Therefore, we must move the electron pairs in the direction of the sulfur atom in order to minimize these charges.
- Minimize formal charges
If necessary, rearrange the electrons by relocating lone pairs to create multiple bonds.
The Lewis structure of SO2 becomes more stable once the electron pair is transferred from the oxygen atom to the sulfur atom.

In the above Lewis dot structure of SO2, each bonding electron pair (:) can also be represented as a single bond (|). As a result, you will have the following Lewis structure for SO2.

Molecular Geometry of SO2
To identify the molecular geometry of sulfur dioxide, we must examine its Lewis structure. Two oxygen atoms are linked to the central sulfur atom. There is also a single pair connected to the sulfur atom. This suggested a bent molecular form.
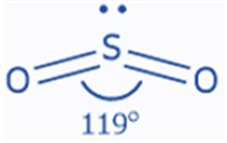
VSEPR theory predicts the structure of the sulfur dioxide molecule. Three substituents (two oxygens and one lone pair) are bonded to the central sulfur atom. Thus, the steric number of sulfur is three. These three groups are approximately 120° apart, indicating a trigonal planar shape. Because one of these substituents is a lone pair, the form will be altered. SO2 has an angular or bent form, with a bond angle of 119°. Both sulfur and oxygen are sp2 hybridized. The VSEPR designation for sulfur dioxide is AX2E1.
| Formula | Shape | Bond Angle |
| AX2 | Linear | 180 |
| AX3 | Trigonal Planar | 120 |
| AX4 | Tetrahedral | 109.5 |
| AX5 | Trigonal Bipyrimidal | 120, 90 |
| AX6 | Octahedral | 90 |
| AX2N | Bent | 120 |
| AX2N2 | Bent | 109.5 |
Video on Lewis Structure of SO2
References
- https://pediabay.com/so2-lewis-structure/
- https://sciedutut.com/so2-lewis-structure/
- https://www.chemicalbook.com/article/how-the-so2-lewis-structure-is-formed.htm
- https://lewistructure.com/so2-lewis-structure/
- https://geometryofmolecules.com/so2-sulfur-dioxide-lewis-structure/