A nonmetal is an element characterized by the absence of metallic attributes. These elements generally lack metallic luster, malleability, ductility, and conductivity of heat and electricity. They also tend to have lower melting and boiling points compared to metals. Nonmetals are predominantly situated on the right side of the periodic table, except for hydrogen, which, despite its position in the upper left corner, displays nonmetallic characteristics under standard temperature and pressure conditions.
Interesting Science Videos
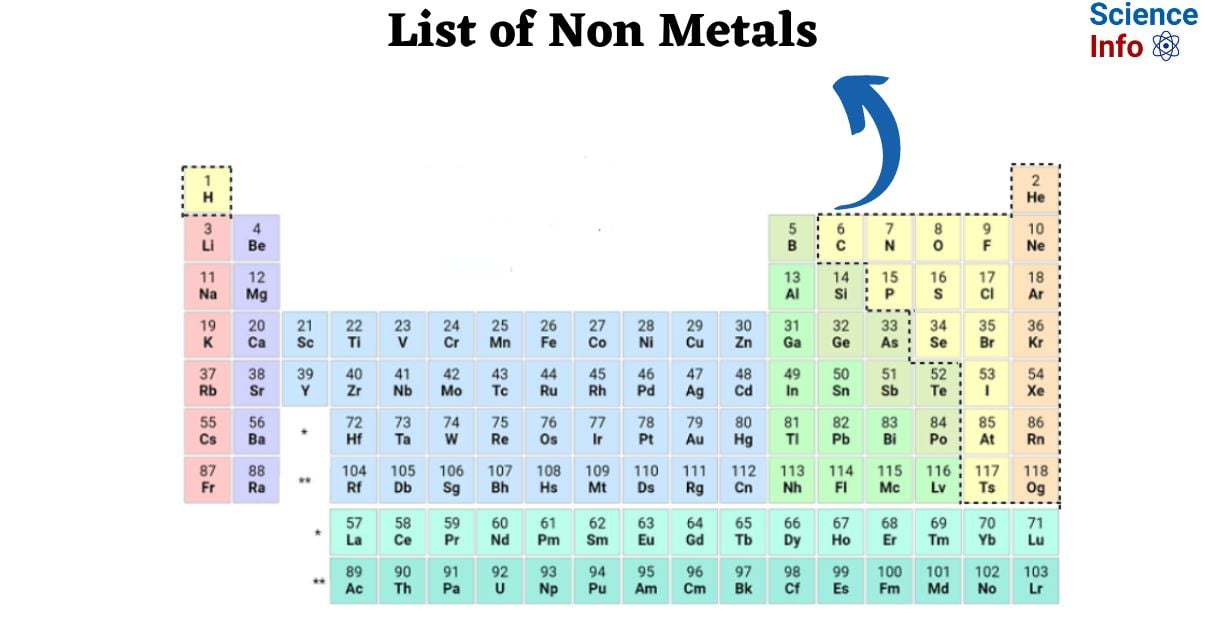
List of Non Metals
| Symbol | Atomic Number | Full Name of Element |
| H | 1 | Hydrogen |
| He | 2 | Helium |
| C | 6 | Carbon |
| N | 7 | Nitrogen |
| O | 8 | Oxygen |
| F | 9 | Fluorine |
| Ne | 10 | Neon |
| P | 15 | Phosphorus |
| S | 16 | Sulfur |
| Cl | 17 | Chlorine |
| Ar | 18 | Argon |
| Se | 34 | Selenium |
| Br | 35 | Bromine |
| Kr | 36 | Krypton |
| I | 53 | Iodine |
| Xe | 54 | Xenon |
| At | 85 | Astatine |
| Rn | 86 | Radon |
| Ts | 117 | Tennessine |
| Og | 118 | Oganesson |
Properties of Non-Metals
Ionization Energies and Electronegativity
- High ionization energies
- Elevated electronegativity
- Tendency to gain electrons in reactions, forming covalent bonds
Atomic Characteristics
- Smaller atom size compared to metals
- Influences various non-metal properties
Electronegativity
- Strong affinity to attract additional electrons
- Preference for holding onto existing electrons
Electrical Conductivity
- Low electrical conductivity
- Contrast with metals in terms of electron donation and conductivity
Physical Properties of Non-Metals
- States at Normal Conditions: Gases, solids, or liquids. Contrasting with metals, especially the exception of mercury
- Solid-State Characteristics: Brittleness. Lack of malleability and ductility, except for carbon fibers
- Ductility and Malleability: Absence of ductility, except for carbon. Lack of malleability, breaking under pressure
- Electrical Conductivity: Low or non-existent electrical conductivity. This is the distinctive feature differentiating non-metals from metals
- Sonorous Properties: Non-sonorous behavior. Lack of resonant sound upon impact, contrasting with metals
- Thermal Conductivity: Poor conductors of heat, similar to their electrical conductivity
Uses of Non Metals
- Hydrogen: Utilized in balloons as a lifting agent and as a highly flammable fuel.
- Oxygen: Used for breathing, steel manufacturing, and medical purposes.
- Carbon: Functions as a fuel, used in steel manufacturing, pencils (graphite form), and various organic compounds.
- Chlorine: Utilized for water purification and contributes to graded plastics and insecticides.
- Helium: Inert gas used in balloons, MRI machines, CT scans, and leak detection in high-vacuum apparatus.
- Nitrogen: Essential for plant growth, used in fertilizers, and in the production of ammonia, nitric acid, and electronic devices. Nitrogen, potassium, and phosphorus play crucial roles in promoting plant growth and development.
- Silicon: A non-metal used in computer chips, solar cells, and the production of fire bricks and transistors.
- Sulphur: Used in gunpowder, matches, rubber manufacturing, and as an insecticide or fumigant.
- Bromine: Applied in pharmaceuticals, water disinfection, flame-retardant materials, dyes, and pesticides.
- Fluorine: Used in refrigeration, air conditioning, insecticides, toothpaste, and the production of Teflon.
- Phosphorus: Utilized in crackers, fine chinaware, special glasses, fireworks, fertilizers, baking powder, and steel production.
- Radon: Applied in hospitals for tumor treatment and disease management.
- Selenium: Used for treating selenium deficiency, essential for proper thyroid and immune system function.
- Iodine: Employed in antibacterial gurgles to prevent throat infections and as a general antiseptic.
References
- https://chemistrytalk.org/periodic-table-metals-and-non-metals/
- https://byjus.com/chemistry/non-metals/
- Emsley, J. (1971). The Inorganic Chemistry of the Non-Metals. Methuen Educational: London. ISBN 0423861204.
- Steudel, R. (1977). Chemistry of the Non-Metals: With an Introduction to Atomic Structure and Chemical Bonding. English edition by F.C. Nachod & J.J. Zuckerman. Berlin: Walter de Gruyter. ISBN 3110048825.
- https://www.thoughtco.com/nonmetals-definition-and-properties-606659
- https://www.thoughtco.com/nonmetals-list-element-groups-606658
- https://sciencenotes.org/list-nonmetals/
