Standard Electrode Potential (Eꝋ) value of three different half-cell types is determined by connecting to a standard hydrogen electrode.
- half-cell for metal / metal ions
- Ion / ion half-cell and
- non-metal / non-metal ion half-cell
Interesting Science Videos
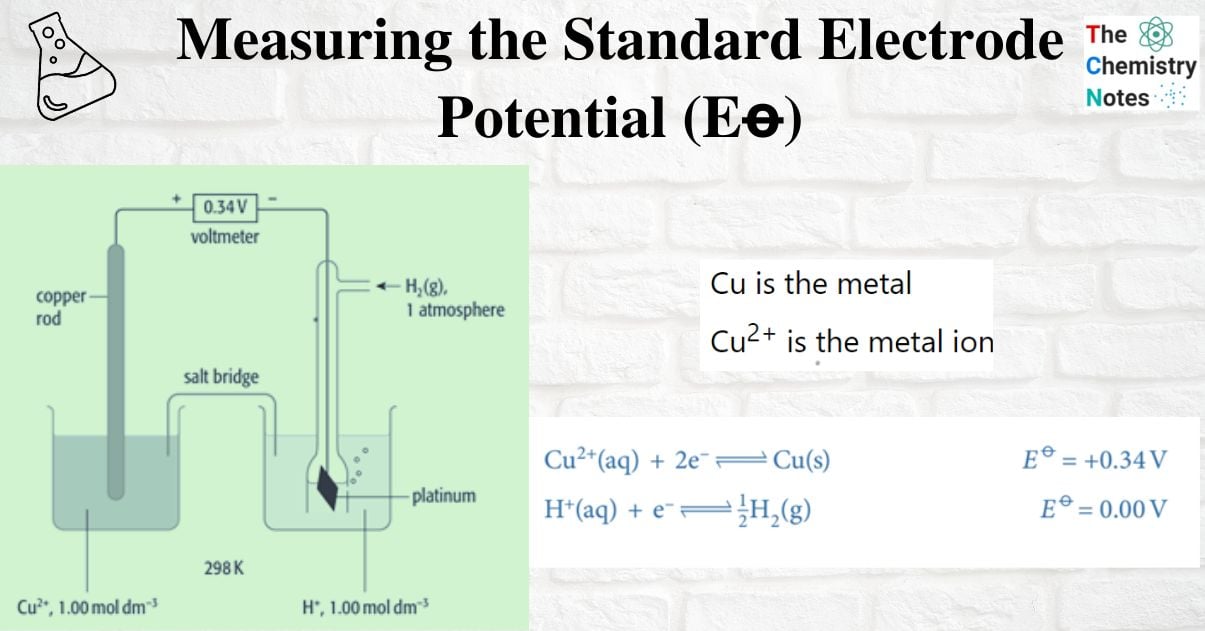
Measuring the standard electrode potential of Metal/metal ion
An example of a metal/metal ion half-cell is the Cu2+ / Cu half-cell
- Cu is the metal
- Cu2+ is the metal ion
![Measuring the standard electrode potential [Cu2+/Cu half-cell]](https://scienceinfo.com/wp-content/uploads/2022/11/image-81.png)
A common hydrogen electrode is connected to the Cu2+/Cu half-cell, and the voltage is measured. The voltage is +0.34V. The positive terminal is copper. The hydrogen electrode is located at the (positive pole) of the negative endpoint. These are the two half-equations:

- The Eꝋ values demonstrate that Cu2+ ions are less difficult to reduce than H+ ions because they have a higher Eꝋ value.
- Cu2+ ions are more likely to gain electrons than H+ ions.
- So, Cu2+ ions will accept electrons from the H+ /H2 half-cell and H2 will lose electrons to the Cu2+/Cu half-cell.
Another example of a metal/metal ion half-cell is the Zn2+ / Zn half-cell
- Zn is the metal
- Zn2+ is the metal ion
![Measuring the standard electrode potential [Zn2+/Zn half-cell]](https://scienceinfo.com/wp-content/uploads/2022/11/image-83.png)
The Zn2+/Zn half-cell has a voltage of -0.76V. The hydrogen electrode is the cell’s positive terminal, and the zinc serves as its negative terminal (negative pole). These are the two half-equations:

- The Eꝋ values show us that Zn2+ ions are more difficult to reduce than H+ ions (they have a more negative Eꝋ value).
- Zn2+ ions are less likely to gain electrons than H+ ions.
- So, Zn will lose electrons to the H+ /H2 half-cell and H+ ions will gain electrons from the Zn2+/Zn half-cell.
Based on these two examples, we can conclude:
- The positive terminal of the cell is where reduction occurs.
As an illustration, in the Zn2+/Zn; H+ /H2 cell:

- Oxidation takes place at the negative terminal of the cell.
For example, in the Zn2+/Zn: H+ /H2 cell:
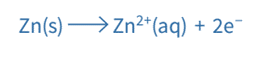
Measuring the standard electrode potential of Non-metal/non-metal ion
Using platinum wire or platinum foil as an electrode, electrical contact with the solution is made in half-cells devoid of metal. At the platinum’s surface, the redox equilibrium is created. Due to its inert nature, the platinum electrode has no impact on the reaction.
Both the element and the aqueous solution containing its ions must come into contact with the platinum.
- An example of a non-metal/non-metal ion is the Cl2/Cl– half-cell
- Cl2 is the non-metal
- Cl– is the non-metal ion
![Measuring the standard electrode potential
[Cl2/Cl– half-cell]](https://scienceinfo.com/wp-content/uploads/2022/11/image-87.png)
[Cl2/Cl– half-cell]
A standard hydrogen electrode is connected to a Cl2/Cl– half-cell. The Cl2/Cl– half-cell has a voltage of 1.36V. As a result, the hydrogen electrode serves as the cell’s negative terminal and the Cl2/Cl– half-cell as its positive terminal. These are the two half-equations:
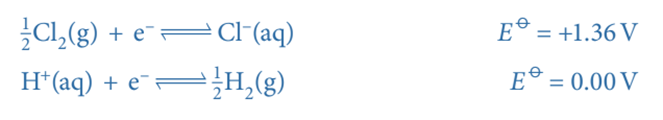
- The Eꝋ values show us that Cl2 molecules are easier to reduce than H+ ions (they have a more positive Eꝋ value).
- Cl2 molecules are more likely to gain electrons than H+ ions.
- So, Cl2 molecules will gain electrons from the H+/ (1/2)H2 half-cell and H2 molecules will lose electrons to the (1/2)Cl2/ Cl– half-cell.
Measuring the standard electrode potential of Ion/Ion half-cell
Half-cells containing ions of the same element in different oxidation states.
Once more, a platinum electrode is utilized to create a half-cell of ions in various oxidation states.
![Measuring the standard electrode potential
[MnO4-/Mn2+ half-cell]](https://scienceinfo.com/wp-content/uploads/2022/11/image-91.png)
[MnO4–/Mn2+ half-cell]
The MnO4–/Mn2+ half-cell is an example of such a half-cell.
MnO4– is an ion with the oxidation state +7 that contains Mn.
The Mn in the Mn2+ ion is in the +2 oxidation state.
The two half-equations for this half-cell, which is connected to a standard hydrogen electrode, are:

- The H+ ions must be present in the half-cell to change the MnO4– ions into Mn2+ ions.
- The positive pole is the MnO4–/Mn2+ – half-cell
- The negative pole is the H+/H2.
Therefore, Eꝋcell = (+ 1.52) – (0.00) = + 1.52 V
Look at the video for the Determination of Standard Electrode potential of Zinc and Copper https://youtu.be/IWCO_vLxotI
Reference
- Hill and Holman (2011) Chemistry in Context, Sixth edition, Nelson Thornes
- Clugston and Flemming (2000) Advanced Chemistry, Oxford University Press
- Cambridge-International-AS-A-Level-Chemistry-9701-EBOOK.pdf
- https://www.savemyexams.co.uk/a-level/chemistry/cie/22/revision-notes/5-physical-chemistry-a-level-only/5-3-principles-of-electrochemistry-a-level-only/5-3-4-measuring-the-standard-electrode-potential/
- https://www.toppr.com/ask/en-np/content/concept/measuring-electrode-potential-203319/

