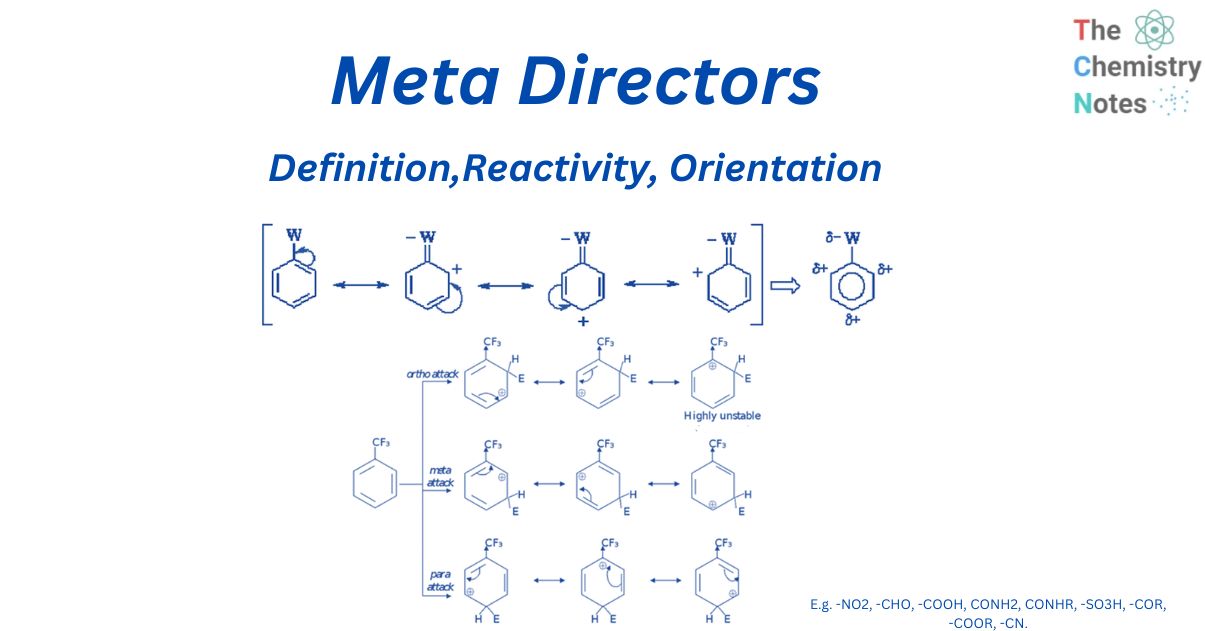
Meta directors are deactivating groups on benzene that direct the electrophile E to the meta position. In comparison to hydrogen, deactivating groups slow down the rate of electrophilic aromatic substitution. Substituents that pull electron density away from nearby carbocations (e.g., CF3, NO2) will avoid leading E to the ortho- or para-position, resulting in the formation of the meta-product.
Example of meta directos include: -NO2, -CHO, -COOH, CONH2, CONHR, -SO3H, -COR, -COOR, -CN.
Interesting Science Videos
What are meta directors?
Meta directors are substituents present on the benzene ring that favor electrophilic attack at the meta position In electrophilic substitution reaction. The reaction occurs in the meta position because these meta directors deactivate the ortho and para positions by taking the electron cloud from them via electron displacement effects, resulting in increased electron density or cloud at the meta position, which allows the coming electrophile to attach to it during the electrophilic substitution reaction.
Electron-withdrawing groups function as deactivators and therefore act as Meta directors. Meta directors are the already-present group on benzene that extracts electrons from the ring either directly through induction or indirectly through resonance.
Species with full positive charges, such as the nitro group or ammonium salts, are found in strongly deactivating groups. Our carbonyls, carboxylic acid derivatives, and related groups are weakly deactivating. Finally, halogens are weakly deactivating groups.
where two positive charges are right next to each other on adjacent atoms. This is a highly unstable and unfavorable thermodynamic condition. As a result, the overall o/p-contributors will be less stable than the meta one. Because they are less stable, they have more energy and form more slowly.
So, electron-withdrawing groups raise activation energy in all places on the benzene ring, but more so in the ortho and para positions than in the meta position. The majority of the products will be meta-substituted because meta-substitution is substantially faster.
Meta-directing nature of electron withdrawing group
Electron withdrawing groups bind electronegative atoms next to the It system and deactivate the aromatic ring by lowering the electron density on the ring via a resonance withdrawing effect. The resonance effect only reduces electron density in the ortho- and para-positions. As a result, these sites are less nucleophilic. So, the system tends to react with electrophiles at the meta sites.
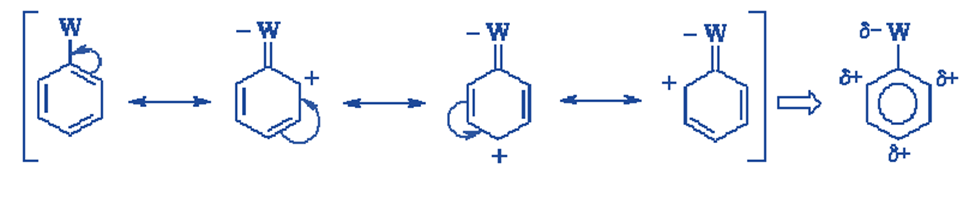
Example: Trifluoromethyl benzene
Because the positive charge is positioned on the ring carbon that contains the electron-withdrawing group, the resonance structures for the arenium ion coming from ortho and para attack that one contributing structure is particularly unstable relative to all the others. In the arenium ion resulting from metaattack, we do not observe any such extremely unstable resonance configuration. This implies that the meta-attack arenium ion should be the most stable of the three.
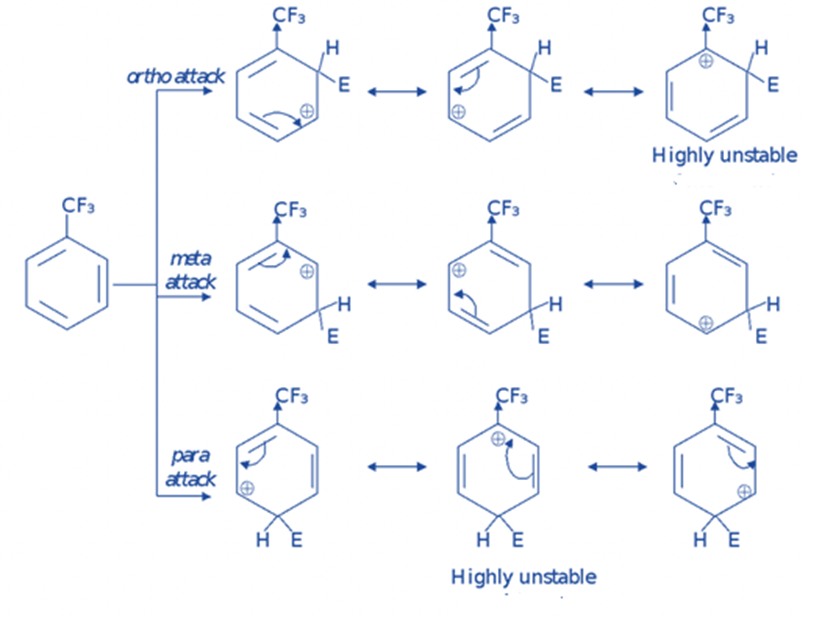
Halogen substituents are unique in that they are deactivating while still directing the incoming electrophile in the ortho-Para direction. The reason for this is that they both exert -I (electronegativity) and +R (lone pair donation) effects. The inductive effect reduces reactivity, whereas the resonance effect controls regiochemistry due to the stability of the intermediates.
Ortho/ Para directing nature of halogens
Although halogens (F, Cl, Br, and I) are ortho, para directing, they deactivate the benzene ring, making additional electrophilic substitutions difficult. This is due to the existence of two opposing effects, namely the + R (or + M) and —I effects.
They are deactivators because they extract electrons from the ring inductively more strongly than they supply electrons via resonance. Inductive electron withdrawal deactivates all ring locations, however, electron donation by resonance partially compensates for this deactivation at the ortho and para positions. The meta is the most inactive state since it cannot benefit from resonance electron donation. As a result, the halogens act as ortho/para directors and deactivators.
References
- Morrison R. T. & Boyd R. N. (1983). Organic chemistry (4th ed.). Allyn and Bacon.
- Smith M. & March J. (2001). March’s Advanced Organic Chemistry: Reactions Mechanisms and Structure (5th ed.). Wiley.
- Ghosh, S.K., Advanced General Organic Chemistry, Second Edition, New Central Book Agency Pvt. Ltd., Kolkatta, 2007.
- Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and Company Ltd, New Delhi, 1992. production of polyesters, polyurethanes, and alkaline resins.
