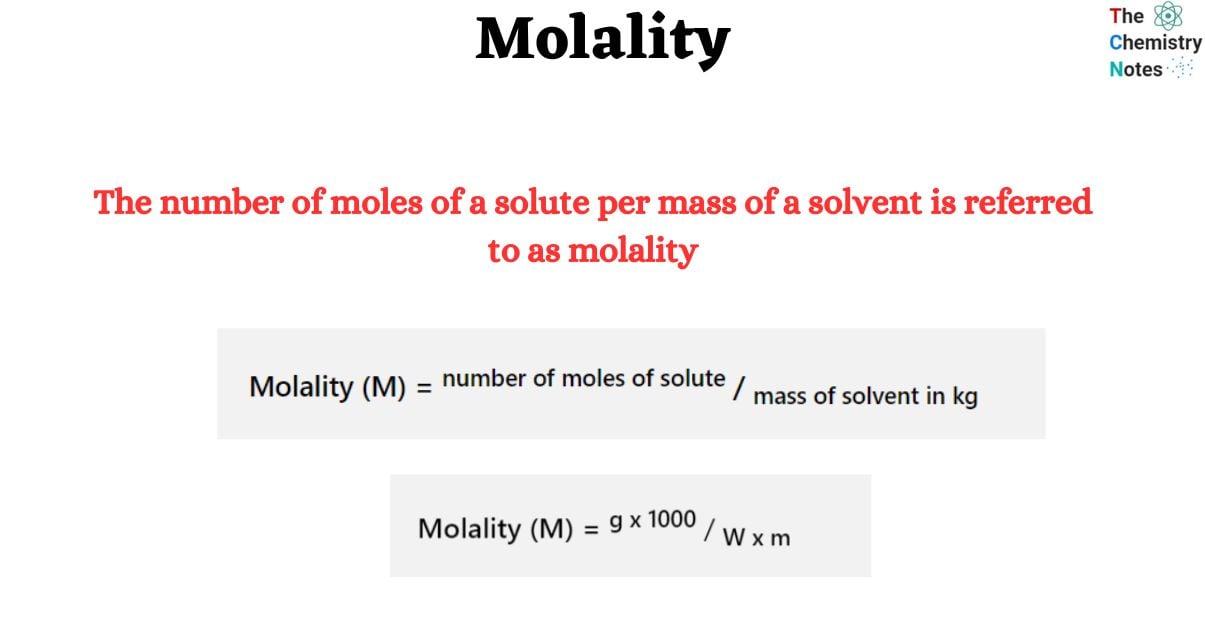
Molality is an important property of solutions. It is used to indicate the concentration of a solute in a solution and is mostly determined by the solvent’s mass. Molality is also known as molal concentration. It is often represented by the letter “m.” The concentration of a solution is defined as the quantity of one ingredient when it is combined with another. The concentration of a chemical solution is measured using both molarity and molality.
The number of moles of a solute per mass of a solvent is referred to as molality, whereas the number of moles of a solute per volume of a solution is referred to as molarity. Molality does not alter with temperature and is utilized for physio-chemical measurements.
Interesting Science Videos
Molality Formula
The System International the ‘Unites of Units, the National Institute of Standards & Technology, the US authority on measurement, considers the term “molal” and consequently the unit sign “m” to be outdated and instead recommends moles/kg or a similar SI unit.
Molality (M) = number of moles of solute / mass of solvent in kgMolality (M) = g x 1000 / W x mMolality varies from molarity solely in the denominator. While molarity is measured in liters of solution, molality is measured in kilos of solvent.
How to Calculate Molality
Molality is mostly used to express the concentrations of solutions as they fluctuate with vapor pressure and temperature. Molality is also utilized to determine the boiling or melting temperature, as well as when working with collative qualities.
- If you know the mass of the solute and solvent in a solution, you can calculate molality fairly effortlessly.
Furthermore, the concentration of a homogeneous solution, or molality, is always constant.
Advantages of Molality
- The benefit of using molality as a measure of concentration is that, as was already stated, the molality is entirely reliant on the mass of the solute and the solvent. This means that changes in temperature or even pressure have no effect on these factors, as opposed to volumetric solutions.
- Furthermore, it is especially advantageous since the molality of one solute in a solution is independent of the presence of other solutes.
Limitations of Molality
- The drawback is that it doesn’t become relevant when there is no pristine substance in an amalgamation. For example, water and alcohol mixes or alloys Any of the compounds might be regarded as the solvent in this case.
Difference Between Molality and Molarity
Molality varies from molarity solely in the denominator. While molarity is measured in liters of solution, molality is measured in kilos of solvent. Here are some differences of molality and molarity:
| Molality | Molarity |
|---|---|
| Molality is defined as the number of moles of a solute divided by the weight of the solvent in kilograms. | The number of moles of solute per kilogram of solvent is referred to as molarity. |
| It remains unaffected by fluctuations in temperature. | It is influenced by variations in the temperature. |
| Molality is measured in mol/kg | The unit of molarity is mol/litre |
Video Reference
References
- https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Introductory_Chemistry_(CK-12)/16%3A_Solutions/16.11%3A_Molality
- https://www.vedantu.com/chemistry/molality
- https://www.geeksforgeeks.org/molality/
- https://byjus.com/chemistry/molality/#:~:text=What%20is%20Molality%3F,two%20components%3B%20solute%20and%20solvent.