Mixed-mode chromatography is a chromatographic technique that involves the interaction between stationary phase with solutes via many interaction modes. Mixed-mode chromatography technology has been quickly increasing because of its advantages over traditional chromatography, such as its high resolution, high selectivity, high sample loading, fast speed, and the possibility to substitute two conventionally matching columns in certain cases.
Multimodal or mixed-mode protein chromatography uses media supports that have been functionalized with ligands that can interact in numerous ways, including ion exchange, hydroxyapatite, affinity, size exclusion, and hydrophobic interactions. The ability to combine various separation approaches improves selectivity in protein purification processes. It combines the high resolution of ion exchange and the high salt tolerance of hydrophobic chromatography. This permits the chromatographic process to be loaded under high salt conditions, resulting in a larger operating space that is ideal for the purification of complex substances.
Interesting Science Videos
What is Multimodal or mixed-mode chromatography?
Mixed mode chromatography (or multimodal chromatography, MMC) is a liquid chromatography technology used to purify proteins and other biological substances. Mixed-mode chromatography has been used successfully in research, process development, and bioprocessing to purify biomolecules that are difficult to separate using traditional chromatography methods. In MM chromatography, ligands immobilized in the resin interact with the target protein molecule through a variety of mechanisms, the most notable of which are size exclusion, and ionic, and hydrophobic interactions.
Multimodal or mixed-mode chromatography can be used to separate proteins, including antibodies. The inclusion of solutes, particularly arginine, increased separation quality and efficiency. Arginine is more effective at facilitating protein elution than salts and protein denaturants like guanidine and urea. The interaction between arginine, proteins, and resin ligands accounts for arginine’s distinct elution impact. Arginine has numerous binding mechanisms for ligands and a higher affinity for protein aromatic residues due to its guanidinium group. These features make arginine suitable for protein elution in multimodal chromatography.
Mixed-mode Chromatography in Purification Process
Multimodal chromatography resins use ligands that can interact in multiple ways, including ion exchange, affinity, size exclusion, and hydrophobicity.
Combining different ways of protein separation can improve purifying selectivity in Mixed-mode chromatography. Enhanced selectivity allows for the removal of contaminants reducing the need for several processing steps. Unlike monomodal chromatography, which separates molecules based on a single feature (e.g. activity, charge, hydrophobicity), mixed-mode chromatography and ligands do not have a known unique interaction with the molecule of interest. Screening mixed-mode resins involves identifying locations on the target molecule that provide optimal selectivity and capacity.
Stationary phase used in multimodal or mixed-mode chromatography
Many chromatographic matrices are built on rigid supports, such as cellulose, agarose, polyacrylamide, or silica gel, that have been modified to provide unique functions on the surfaces. When dealing with substances that have multiple functional groups, such as proteins, peptides, nucleic acids, and amino acids, which are frequently present in biological samples, mixed-mode chromatography will behave differently from single-mode chromatography.
Mixed-mode stationary phases must be designed and synthesized depending on the specific structural properties of distinct compounds to improve solute retention, selectivity, and separation capability. Furthermore, the mixed-mode stationary phase’s diversity is determined by the structure and properties of the analyte.
Type 1: A mixed-mode stationary phase is formed by fitting two types of stationary phase particles into a single column. However, the main disadvantages of this method are the non-homogeneity of the stationary phases and the limited batch-to-batch reproducibility.
Type 2: The stationary phase’s surface is changed with a combination of ligands of various chemistries. This is a second-generation technique, although its drawbacks are comparable to those of Type 1. As a result, Types 1 and 2 are rarely employed due to performance limits.
Types 3 and 4: The third-generation mixed-mode phases, embedded (Type 3) and tipped ligands (Type 4), increase the stationary phase’s repeatability and homogeneity. The embedded ligands’ functional groups (polar or ionic groups) lie close to the pore surface, while their hydrophobic sections extend into the mobile phase. In contrast, tipped ligands feature functional groups at the ends of their hydrophobic chains.
Combinations of separation modes in Mixed-mode chromatography
- Reverse phase ione exchange stationary phase (RP-IEX)
Polar substances, such as biologically active molecules, natural products, and drug metabolites with several functional groups, are typically weakly retained in the reversed-phase, resulting in poor separation. Combining hydrophobic and ion-exchange mechanisms in mixed-mode stationary phases improves the selectivity and retention of both hydrophobic and polar molecules.
- Reverse phase hydrophilic stationary phase
Adding hydrophilic groups to the hydrophobic stationary phase enhances the HILIC characteristics of RPLC stationary phases. Short alkyl phases in RPLC retain polar solutes like peptides and proteins more effectively than long alkyl chain stationary phases. Polar organic solvents (e.g., THF, CH3CN, and CH3OH) used in RPLC can significantly improve selectivity, making them suitable for 2DLC.
RP-HILIC mixed-mode stationary phases have shown advantages for the separation of hydrophobic and hydrophilic substances, particularly proteins. This combination is analogous to combining the HILIC properties with the reversed-phase properties to analyze complex compounds and matrices with a wide variety of polarity in one run. The ligands consist of hydrophobic and polar groups. The hydrophobic sections can be alkyl or aromatic groups, whereas the hydrophilic parts can be charged or neutral functional groups, such as diol, amide, cyano, and ionic.
- Hydrophilic ion-exchange stationary phases
The combination of hydrophilic and ion-exchange groups provided significant advantages when analyzing charged polar substances. These mixed-mode phases have unique selectivity, superior retention efficiency, and a broader variety of applications than any single-mode phase for peptide analysis.
HILIC/CEX
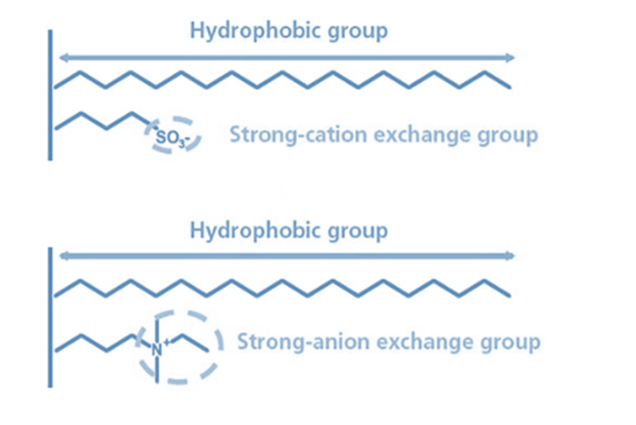
This chromatographic technique uses cation exchange and hydrophobic interaction to separate and purify molecules based on their charge and hydrophobicity. This method includes both hydrophobic groups and cation exchange ligands in the stationary phase. Cation exchange ligands are primarily composed of negatively charged functional groups, such as sulfonic or carboxylic acid groups, that interact with positively charged analytes via electrostatic interactions. Additionally, hydrophobic groups interact with analytes through hydrophobic interactions.HILIC/CEX has several advantages over RPLC for peptide separation, including superior selectivity, higher separation efficiency, and a wider range of applications.
HILIC/AEX
Anion Exchange chromatography isolates analytes based on their charge interactions with the stationary phase’s positively charged functional groups. In this stationary phase, negatively charged ions remain intact, whereas neutral or positively charged species are eluted. HILIC/AEX’s stationary phase comprises both hydrophilic and anion exchange functional groups. This combination enables the simultaneous exploitation of hydrophilic and charge-based interactions, resulting in improved selectivity for complex analytes with various characteristics, such as polar molecules with different charges.
The HILIC/AEX stationary phase for capillary LC of polar analytes demonstrated high selectivity for peptides, basic, and acidic analytes. The separation of basic chemicals, including nucleic acids, bases, and nucleosides, was effective without peak tailing. HILIC/AEX amino columns outperformed other methods for separating tiny molecular medicines.
- Inclusion hydrophobic mixed-mode
Inclusion hydrophobic mixed-mode ligands consist of hydrophobic portions and cavities, cages, or cryptates that form an inclusion complex with the analytes. Thus, this technique has multivalent effects that comprise both inclusion complexation and hydrophobic contacts. For example: Crown ether immobilized on a solid matrix.
- Inclusion hydrophilic mixed mode
In this method, the ligands consist of a cavity, cage, or cryptate, as well as a polar group. The binding of crown ethers to primary amines is one example of this chromatographic process. A polar organic solvent mobile phase can improve hydrogen bonding between the principal amines and crown ether, resulting in stronger inclusion effects.
- Size Exclusion- Ion Exchange(SEC/IEC) Mixed mode
In an ideal SEC, analytes are separated purely by their size.
Non-ideal SEC requires additional interactions to retain analytes. The SEC/IEC mode utilizes the SEC’s non-ideal properties. Low ionic strength leads to poor hydrophobic interactions in proteins. Electrostatic effects can considerably impact retention, and an SEC column can function as a weak ion exchanger.
Advantages of mixed-mode chromatography
- Mixed-mode chromatography has excellent selectivity. A reversed-phase (RP) or anion-cation exchange (ACE) column may separate positive, negative, and neutral compounds in a single run.
- It has a high loading capacity. For example, the loading capacity of ACE/hydrophilic interaction chromatography (HILIC) is 10-100 times more than that of RPLC, presenting a new challenge for the development of semi-preparative and preparative chromatography.
- High throughput approaches, such as design-of-experiment plate screening and, more recently, improved mechanistic modeling, can be used to predict the best mobile phase parameters for a given biomolecule separation.
- Mixed-mode chromatography provides for a broader range of ligand interactions, making it especially effective for purifying highly charged or polar compounds, as well as those with salt or pH sensitivity, which can make separation using reverse phase, ion exchange, or affinity liquid chromatography difficult.
- The concurrent modulation of multimodal interactions can produce enhanced biomolecule resolution, a useful feature not only in bioprocessing but also in HPLC applications for analytical scale bio-separations and impurity analysis.
Comparison between Multimodal and monomodal approaches
- One mixed-mode column with efficiencies equivalent to or greater than the corresponding single-mode stationary phases can replace two or more single-mode columns, reducing waste and material usage.
- Mixed-mode chromatography provides numerous interaction modes, increasing adaptability and selectivity for a wide range of target molecules, including bi-specific antibodies, acid- and salt-sensitive antibodies, and ADCs. Multimodal ligands may have stronger target binding capacity than standard monomodal approaches, resulting in more effective capture and purification.
- Mixed-mode chromatography provides a gentle approach to the purification of sensitive compounds, reducing protein denaturation and aggregation during the process. The capacity to purify several targets in a single step reduces the number of purification stages, saving money, time, and resources.
References
- https://www.sciencedirect.com/science/article/abs/pii/S0021967311015007
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3187938/
- https://pubmed.ncbi.nlm.nih.gov/29065828/
- https://www.slideshare.net/kbibiopharma/multimodal-chromatography-60923405
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2774265/
- https://www.bio-rad.com/en-np/applications-technologies/introduction-multimodal-or-mixed-mode-chromatography?ID=LUSN9AKG4
- https://www.slideshare.net/kbibiopharma/a-comparison-of-multimodal-chromatographic-resin-protein-binding-selectivity
