N-Bromosuccinimide (NBS) is a useful organic chemistry reagent. NBS has traditionally been thought of as a convenient supply of either cationic bromine for electrophilic addition processes or a bromine radical for radical substitution.
N-bromosuccinimide is a useful bromine source for both radical substitution and electrophilic addition processes. NBS provides various benefits over molecular Bromine for radical substitution processes, while 1, 3-Dibromo-5,5-dimethylhydantoin is another useful reagent.
Interesting Science Videos
N-Bromosuccinimide: Brominating agent
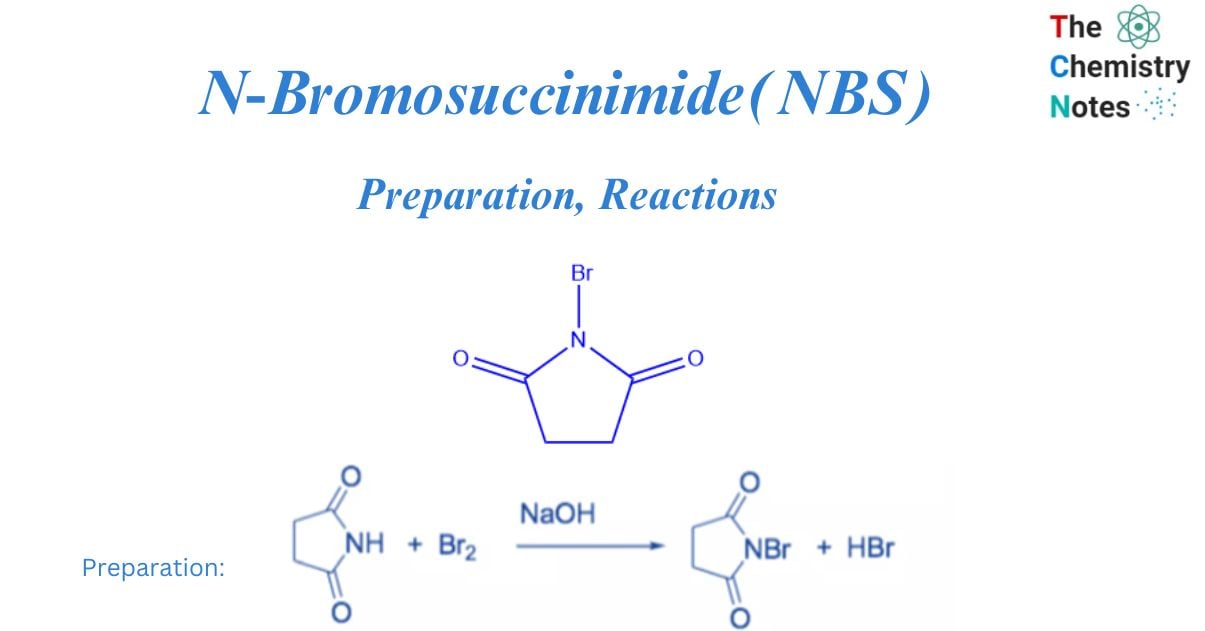
N-bromosuccinimide (NBS) is an easy way to get bromine for radical substitution and electrophilic addition reactions. It is made by combining succinimide and bromine in the presence of a NaOH solution.
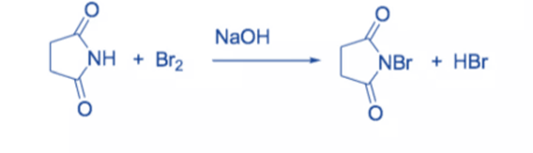
To avoid decomposition, the colorless solid is rinsed with water and recrystallized from hot water before being stored in a refrigerator and protected from moisture.
One advantage of utilizing NBS over bromine is that it is easier and safer to handle; nonetheless, the solid is an irritant, and bromine may be produced during some processes. As a result, measures should be taken to avoid inhaling the powder and coming into touch with the skin. All activities with this reagent should be carried out in a well-ventilated fume hood. Furthermore, because NBS reactions are often highly exothermic, large-scale operations (>0.1 mol) should be cautiously approached.
Reactions of N-Bromosuccinimide
Allylic bromination
The replacement of hydrogen on a carbon close to a double bond (or aromatic ring, in which case it is called benzylic bromination) is known as allylic bromination. In these situations, NBS is employed as an alternative for Br2 since Br2 tends to react with double bonds to generate dibromides. NBS has the benefit of providing a low-level concentration of Br2, and bromination of the double bond does not compete as strongly.
Under reflux circumstances, alkenes react with NBS in dry CCl4 to produce allyl bromide. Light or peroxide initiates the process. Although there are other reagents available for the bromination of the allylic C-H bond of alkenes, NBS is the most widely utilized. The reaction is known as Wohl-Zigler bromination.
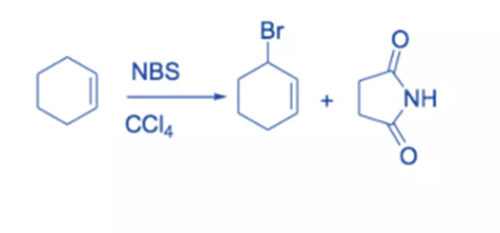
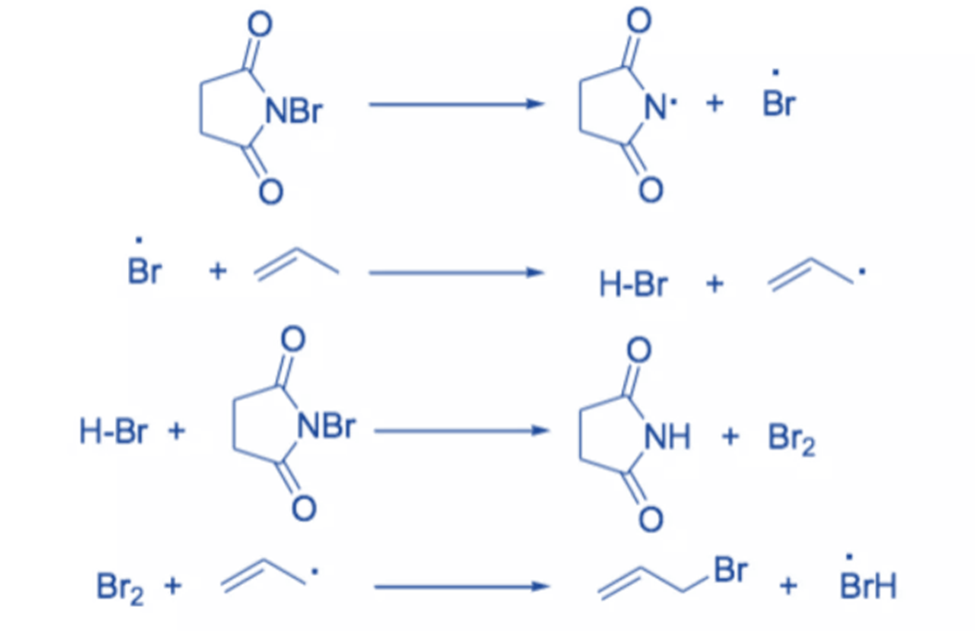
The reaction involves a free radical mechanism. Small quantities of Br radical start the reaction. NBS’s function is to provide a continuous low concentration of molecular bromine. The removal of allylic or benzylic hydrogen by a Br radical results in the formation of a resonance-stabilized allyl or benzyl radical. Selective bromination takes place because the intermediate leading to the product is stabilized by resonance.
Carbonyl derivative bromination
NBS can brominate carbonyl compounds via a radical pathway or acid catalysis. NBS, for example, can brominate hexanoyl chloride in the alpha position via acid catalysis.
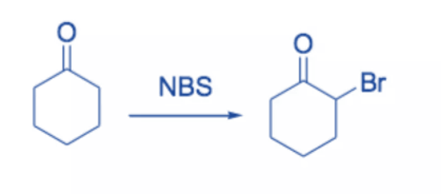
Because it is high-yielding and has minimal byproducts, the reaction of enolates, enol ethers, or enol acetates with NBS is the favored method of α-bromination.
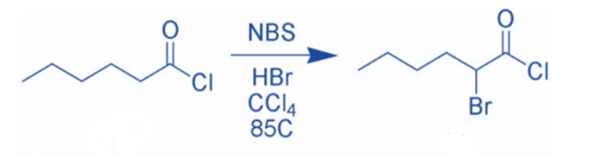
Ketones with enolizable hydrogen can be brominated at the α -position. The reaction most likely involves the addition of Br2 to the enol form of the carbonyl derivatives, followed by the elimination of HBr to produce the α -bromoketone.
Addition to alkene
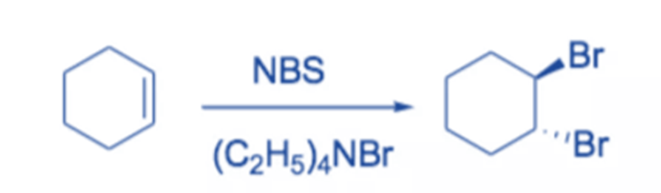
In polar solvents, NBS is frequently employed as a source of electrophilic bromine.
For example, in the presence of tetraethyl-ammonium bromide, cyclohexene combines with NBS to produce trans-I,2-dibromocyclohexane in high yield.
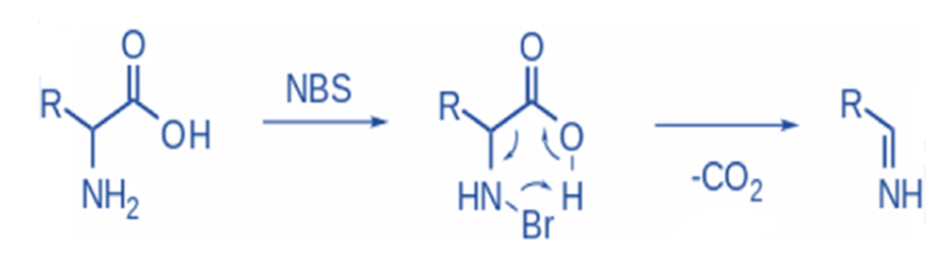
Oxidative decarboxylation of α –amino acids
N-Bromosuccinimide electrophilically brominates the amine, which is then decarboxylated, and an imine is released. Further hydrolysis will result in the formation of an aldehyde and ammonia (non-oxidative PLP-dependent decarboxylation).
References
- Morrison R. T. & Boyd R. N. (1983). Organic chemistry (4th ed.). Allyn and Bacon.
- Smith M. & March J. (2001). March’s Advanced Organic Chemistry: Reactions Mechanisms and Structure (5th ed.). Wiley.
- Ghosh, S.K., Advanced General Organic Chemistry, Second Edition, New Central Book Agency Pvt. Ltd., Kolkatta, 2007.
- https://www.organic-chemistry.org/chemicals/oxidations/n-bromosuccinimide-nbs.shtm.
- https://www.masterorganicchemistry.com/2011/06/10/reagent-friday-nbs-n-bromo-succinimide/
