Nobelium is a synthetic chemical element with an atomic number of 102 and is represented by the symbol ‘No’ in the periodic table. It is silvery in appearance and belongs to the f-block of period 7 of the periodic table. Nobelium was identified as the tenth synthetic trans-uranium element in the actinide series. Similar to other elements in the actinide class, mendelevium exhibits significant radioactivity. A. Ghiorso, T. Sikkeland, J.R. Walton, and G.T. Seaborg employed a new double-recoil technique to unequivocally detect and identify Nobelium in Berkeley in April 1958. Nobelium, like all elements with an atomic number more than 100, can only be created in particle accelerators by blasting lighter elements with charged particles.
Interesting Science Videos
Discovery of Nobelium
- Several groups worked to synthesize nobelium during the 1950s and 1960s.
- At Moscow’s Institute of Atomic Energy in 1956, a team of scientists led by Georgy Flerov blasted plutonium with oxygen to produce isotope-252 of the new element. However, no official declaration was made at that time.
- Isotope-253 was created in 1957 at the Nobel Institute of Physics in Stockholm by bombarding curium with carbon, although Albert Ghiorso claimed to have created isotope-254 in 1958. Russians questioned all of these stories.
- From 1962 to 1963, the Russian Joint Institute of Nuclear Research at Dubna synthesized isotopes 252 to 256 of the new element, but Ghiorso and his team continued to claim they were the first to find it. After years of debate, the International Union of Pure and Applied Chemists ruled in favor of the Russian side.
- The term nobelium was given in honor of Alfred Nobel, the inventor of dynamit
- The IUPAC officially recognized element 102 as nobelium in 1997.
Occurrence of Nobelium
- Nobelium is not found naturally on Earth; it is a synthetic element.
- Nobelium is produced artificially through nuclear reactions involving heavy isotopes.
- It is commonly synthesized by bombarding target materials containing heavy actinide elements with accelerated ions.
- Uranium or curium targets are often irradiated with high-energy particles, like neon or carbon ions, to create nobelium.
- The new isotopes of nobelium generated through these reactions can be isolated and studied for scientific research purposes.
Elemental Properties of Nobelium
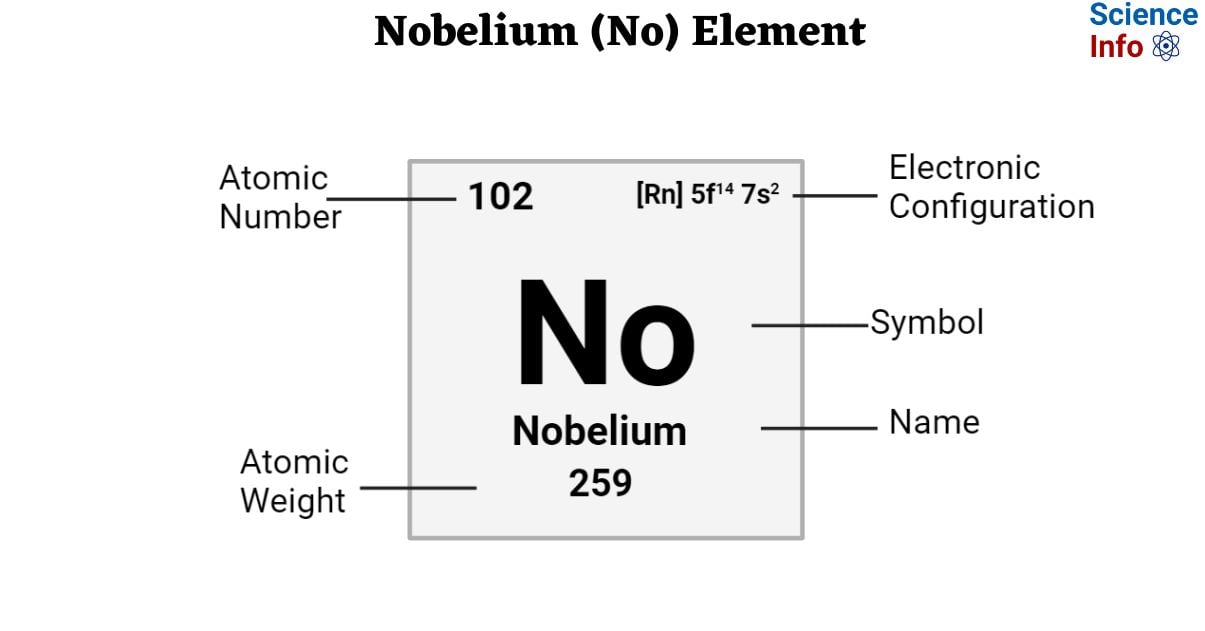
| Electronic Configuration | [Rn] 5f14 7s2 |
| Atomic Number | 102 |
| Atomic Weight | 259 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | Actinides, 7, f-block |
| Density | – |
| Ionic radius | – |
| Van der Waals radius | – |
| Electron shells | 2, 8, 18, 32, 32, 8, 2 |
| Electrons | 102 |
| Protons | 102 |
| Neutrons | 157 |
Isotopic Information of Nobelium
Nobelium exhibits various isotopes, each with distinct half-lives, contributing to our understanding of its nuclear properties.
Isotope and Half-Lives:
- 253No: Half-life of 1.6 minutes
- 254No: Half-life of 51 seconds
- 255No: Half-life of 3.1 minutes
- 257No: Half-life of 25 seconds
- 259No: Half-life of 58 minutes
Stable Isotope: Among the produced isotopes, nobelium-259 stands out as the most stable, with a half-life of approximately 58 minutes. Radiochemists, utilizing traces of nobelium-259, have demonstrated the element’s presence in aqueous solutions in both the +2 and +3 oxidation states.
Decay Pathways:
- Nobelium-259, being the most stable isotope, undergoes decay:
- Through alpha decay, transforming into fermium-255.
- Through electron capture, resulting in mendelevium-259.
- Through spontaneous fission, contributing to its decay pathways.
Physical Properties of Nobelium
- Nobelium, a radioactive metal, is the second-to-last element in the actinide series.
- With an atomic mass of 259 units, nobelium is relatively heavy at the atomic level.
- The predicted melting point is 827°C, indicating when it changes from solid to liquid.
- The boiling point of nobelium is currently unknown.
- Its estimated density at 20°C is around 9900 in S.I. units, representing mass per unit volume.
- Nobelium in metallic form has not been produced in large quantities, posing challenges for experiments.
- The understanding of nobelium relies on predictions from similar elements in the lanthanide and actinide groups.
- It is expected to have a face-centered cubic crystal structure, similar to certain elements in its family.
- Scientific exploration continues to delve into properties like specific heat capacity, electrical conductivity, and reactivity.
Chemical Properties of Nobelium
- When dissolved in water, nobelium takes on the form of divalent cations.
- The element readily exhibits a +2 oxidation state. Originally thought to be unique, nobelium naturally possesses a +3 oxidation state since it belongs to the actinide class.
- It is difficult to achieve a +3 oxidation state in nobelium.
- The majority of research on nobelium’s chemical behavior focuses on aqueous solutions since they provide information on its reactivity.
- The capacity of nobelium to form complexes with chloride ions is similar to that of barium.
- Important chemical parameters like electrode potential, enthalpy, and Gibbs energy are theoretical predictions for which experimental validation is still lacking.
Production of Nobelium
The production of nobelium is a complex and hazardous process conducted with precision. Carbon-12 ions serve as bombarding agents, forcibly directed at the target nucleus (Californium-249 or another transuranium atom) within a cyclotron.
Determining the atomic radius of nobelium involves presuming its electron clouds to resemble a spherical atom. Parameters defining the atomic radius include Van der Waals Radius, Ionization Radius, Covalent Bonding Radius, and Metallic Radius. Van der Waals Radius is half the closest distance between non-bonded elemental atoms in proximity. Ionization Radius, in an ionic linkage, is half the distance between the nuclei of two ions. Covalent Bonding Radius, in a covalent linkage, is represented by the atomic radius of one atom. Metallic Radius, within a crystal with metallic bonds, is half of the internuclear distance.
Production Methods:
- Nobelium, with an atomic number exceeding 100, is exclusively produced in a particle accelerator (e.g., a cyclotron).
- Bombardment of actinide targets (uranium, plutonium, curium, californium, and einsteinium) is utilized for all nobelium isotopes except nobelium-262.
- Nobelium-262 is produced as the daughter isot of lawrencium-262.
- The prevalent isotope, nobelium-255, is synthesized by bombarding californium-249 or curium-248 using carbon-12 ions.
Uses of Nobelium
Nobelium, being an artificially created metal, falls within the category of rare earth metals. Due to its limited abundance, essential characteristics of nobelium are still undiscovered, positioning it as a subject of extensive research.
The predominant utilization of nobelium resides in research laboratories, where ongoing investigations aim to unveil its novel properties. Their inherent instability often leads to decomposition into alternative forms, and various isotopes contribute to diverse scientific applications. While its nuclei are challenging to detect, the element’s significance in exploring fundamental scientific principles remains unparalleled.
Health Effects of Nobelium
- Nobelium is considered biologically inactive, indicating minimal direct impact on living organisms.
- Being a radioactive element, nobelium’s emission of ionizing radiation poses potential health hazards.
- Limited applications and uses in research contexts result in minimal human exposure to nobelium.
- The undetectable nature of nobelium’s nuclei further reduces direct interactions with biological entities.
- To reduce any health concerns, researchers using nobelium must follow strict safety standards.
- Ongoing monitoring and regulation of occupational exposure are essential for those involved in nobelium-related research.
- Comprehensive studies on the health effects of nobelium are limited due to its rarity and restricted applications.
- Continued research is necessary to enhance our understanding of potential health implications associated with nobelium.
Environmental Effects of Nobelium
- Nobelium, with its short half-life, exerts negligible long-term effects on the environment.
- The absence of nobelium in the Earth’s crust indicates its natural non-occurrence in terrestrial ecosystems.
- Due to its instability, nobelium easily decomposes into other elements, preventing prolonged environmental impact.
- Nobelium’s utilization primarily in controlled research environments minimizes the likelihood of uncontrolled environmental release.
Video on Nobelium
References
- https://www.elementalmatter.info/element-nobelium.htm
- https://www.livescience.com/40416-facts-about-nobelium.html
- https://periodic-table.com/nobelium/
- https://chemicalengineeringworld.com/nobelium-element-properties-and-information/
- https://byjus.com/chemistry/nobelium/
- https://education.jlab.org/itselemental/ele102.html
- https://www.vedantu.com/chemistry/nobelium
- https://www.azom.com/article.aspx?ArticleID=7933
- https://www.radiochemistry.org/periodictable/elements/102.html
- https://www.rsc.org/periodic-table/element/102/nobelium

