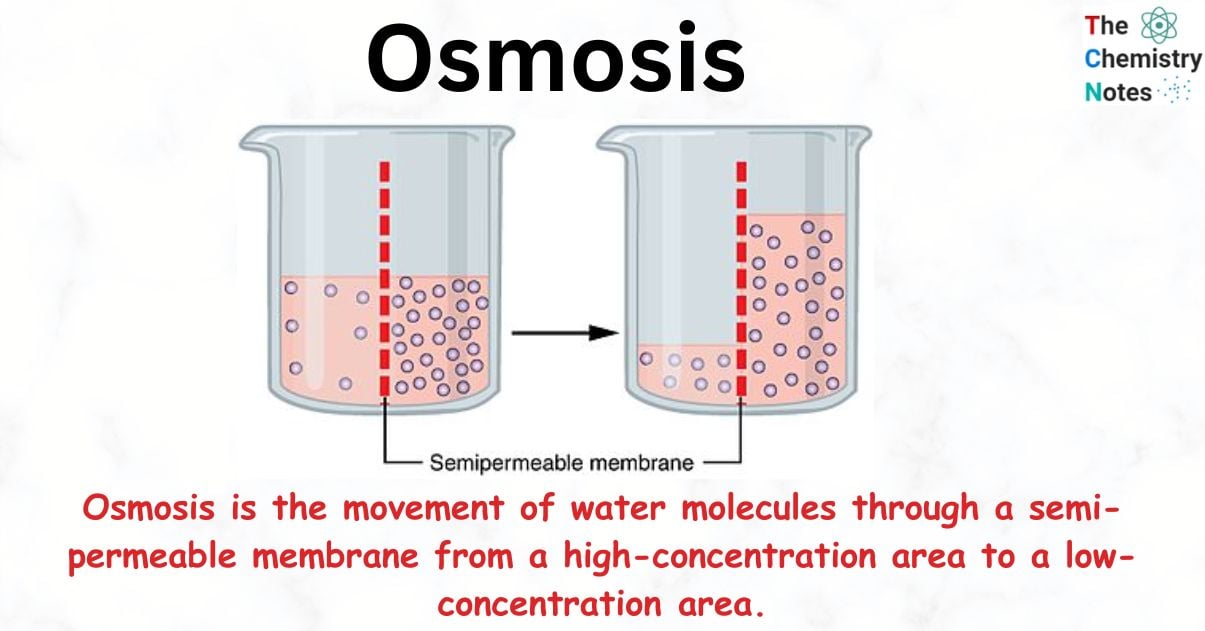
Osmosis is the movement of water molecules through a semi-permeable membrane from a high-concentration area to a low-concentration area. Osmosis happens across the cellular membrane to protect a cell from becoming flaccid (not enough water) or turgid (too much water).
Interesting Science Videos
What is Semi-permeable membrane?
Semi-permeable membranes are those that permit the passage of solvent molecules but not solute particles.
The semi-permeable component might be either natural or artificial. Natural semi-permeable membranes include the membranes that envelop plant and animal cells, the bladder of a pig, and so on. The gelatinous precipitate of inorganic compounds such as calcium phosphate and copper ferrocyanide may be employed to create artificial semi-permeable membranes.
What is Osmosis?
Osmosis occurs when a solution is separated from a solvent by a semipermeable membrane, allowing solvent molecules to flow through the barrier and into the solution. This phenomenon is referred to as osmosis.
The semi-permeable membrane can likewise be used to achieve the same effect. In such instances, the solvent molecules can move across the semi-permeable barrier from a lower concentration solution to a higher concentration solution.
In other words, osmosis may be described as the spontaneous transport of solvent molecules onto or from a solution across a semi-permeable membrane.
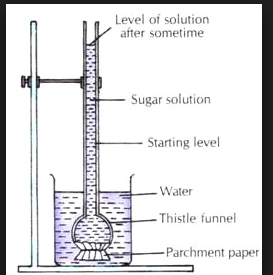
To illustrate the phenomena, take an inverted thistle funnel and bind its mouth shut with a semi-permeable membrane, such as an animal bladder. It is then partially filled with the concentrated solution sugar and stored in a breaker holding pure solvent (water). Now osmosis occurs, in which the solvent molecules move across a semi-permeable membrane from the pure solvent to the sugar solution. As a result, the water rises to a specific height in the narrow tube of the funnel, as indicated in the image.
This phenomena continues until the hydrostatic pressure of the solution inside the funnel simply stops the flow of water from the beaker. Solute particles, like gas molecules, can diffuse from solution into pure solvent or from a higher concentration to a lower concentration to achieve a uniform concentration. This natural phenomena of solute particle movement is known as diffusion.
Diffusion is the flow of a fluid from a higher concentration area to a lower concentration area.
| Osmosis | Diffusion |
| In osmosis, the flow of solvent molecules takes place into the solution through the semi-permeable membrane. | In diffusion, the flow of both solute and solvent molecules takes place and semi-permeable membrane is not required. |
| Solvent flows into solution or the solution of lower concentration to the solution of higher | Solution flow from higher concentration to lower concentration to get the uniform concentration. |
Types of Osmosis
There are two kinds of osmosis:
- Endosmosis
- Exosmosis
Endosmosis occurs when a material is immersed in a hypotonic solution and the solvent molecules travel into the cell, causing the cell to become turgid or undergo deplasmolysis. This is referred to as endosmosis.
When a material is immersed in a hypertonic solution, the solvent molecules travel outside the cell, causing the cell to become flaccid or suffer plasmolysis. This is referred to as exosmosis.
Osmotic Types of Solution
There are three types of solutions:
- Isotonic Solution
- Hypertonic Solution
- Hypotonic Solution
Isotonic Solution
An isotonic solution contains the same number of solutes as another solution. For example, an isotonic cell to the surrounding solution indicates that both the internal fluid and the external fluid have the same osmotic pressure and water potential. In this instance, there will be no net flow of water molecules between the cell and the surrounding fluid.
Hypotonic Solution
A hypotonic solution is one that has a lower osmotic pressure (or contains fewer solutes) than another solution. In this situation, water travels toward the less concentrated zone or toward the more concentrated part to dilute the solution. When the fluid surrounding the cell is hypotonic, for example, water will migrate across the membrane toward the more concentrated solution inside the cell.
Hypertonic Solution
A hypertonic solution appears to be the inverse of a hypotonic solution. A hypertonic solution has more solutes and less water than the other solution. When a cell is submerged in a hypertonic solution, water will exit the cell to dilute the solution outside.
Illustration of Osmosis
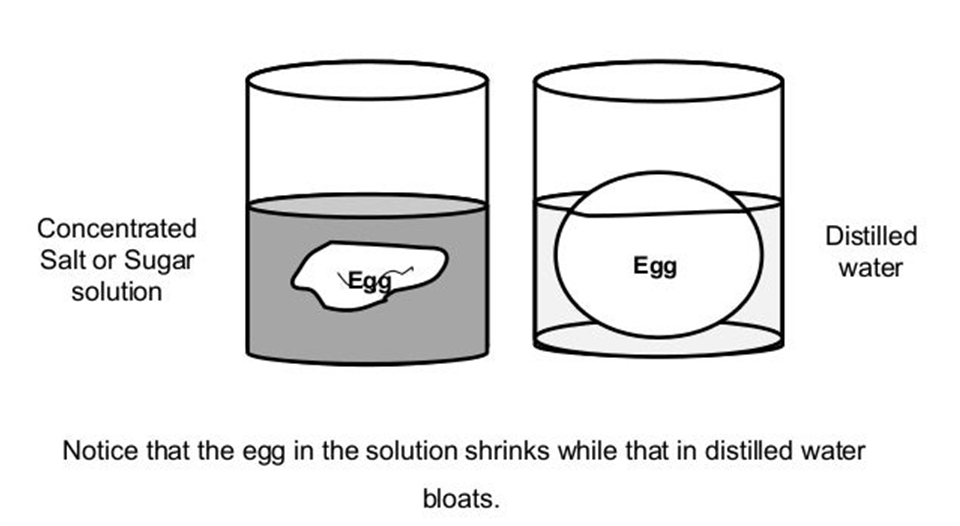
To begin, two eggs of equal size are selected and their outer shells are removed. One is now immersed in pure water, while the other is immersed in saturated NaCl solution. After a few hours, the egg in the water swells while the egg in the salt solution shrinks. That is because, in the first scenario, water enters through the membrane into the concentrated egg material, causing swelling, but in the second situation, water exits the egg material into a more concentrated salt solution, resulting in uniform concentration. As a result, in the second situation, the egg shrinks. The osmosis process can be illustrated in the following egg experiment.
Importance of Osmosis
- Osmosis affects the movement of nutrients and the expulsion of metabolic waste.
- It absorbs water from the earth and transports it to the plant’s higher sections via the xylem.
- By preserving the equilibrium between the amounts of water and intercellular fluid, it stabilizes the environment inside a living thing.
- Despite the ongoing water loss via transpiration, it is a mechanism by which plants retain their water content.
- Osmosis causes cell turgor, which controls how much movement is allowed for plants and plant components.
Osmotic Pressure
Osmotic pressure is the pressure necessary to prevent water from diffusing through a membrane by osmosis. It is determined by the concentration of the solute. Water diffuses from the area of lower concentration to the area of greater concentration. When the concentrations of the compounds in the two locations in contact differ, the substances will disperse until the concentration is uniform throughout.
After a while, the level of the solution on the concentrated solution becomes constant. When the hydrostatic pressure created by concentrated solution counterbalances the flow of pure solvent into the solution, this stage is reached. Thus, osmotic pressure may be described as the hydrostatic pressure imposed by a solution that simply prevents solvent from passing through a semi-permeable barrier.
References
- Borg, Frank (2003). “What is osmosis? Explanation and understanding of a physical phenomenon”. arXiv:physics/0305011
- https://byjus.com/biology/osmosis/
- Hammel, H.T.; Scholander, P.F. (1976). Perspectives on the Mechanism of Osmosis and Imbibition In: Osmosis and tensile solvent. Springer-Verlag, Berlin, Heidelberg, New York.
- https://www.britannica.com/science/osmosis
- https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Introduction_to_General_Chemistry_(Malik)/05%3A_Solutions/5.05%3A_Osmosis#:~:text=Osmosis%20is%20the%20passage%20of,required%20in%20the%20biological%20systems.
