Oxidation and reduction reactions are as common and well-known as fire, the oxidation and dissolution of metals, the discoloration of fruit, and respiration and photosynthesis—fundamental life processes. Chemical reactions come in a variety of forms. Some of these reactions, like the burning of gasoline and the rusting of iron, are things we can see in everyday life. The foundation for the creation of various compounds is a variety of crucial industrial reactions.
Chemical reactions involve the transfer of electrons from one chemical substance to another. These electron-transfer reactions are termed “oxidation-reduction reactions” or “redox reactions.” Energy changes as heat, light, electricity, and other phenomena accompany these reactions. The oxidation and reduction reactions also involve the addition of oxygen or hydrogen to different substances.
Interesting Science Videos
What is Oxidation?
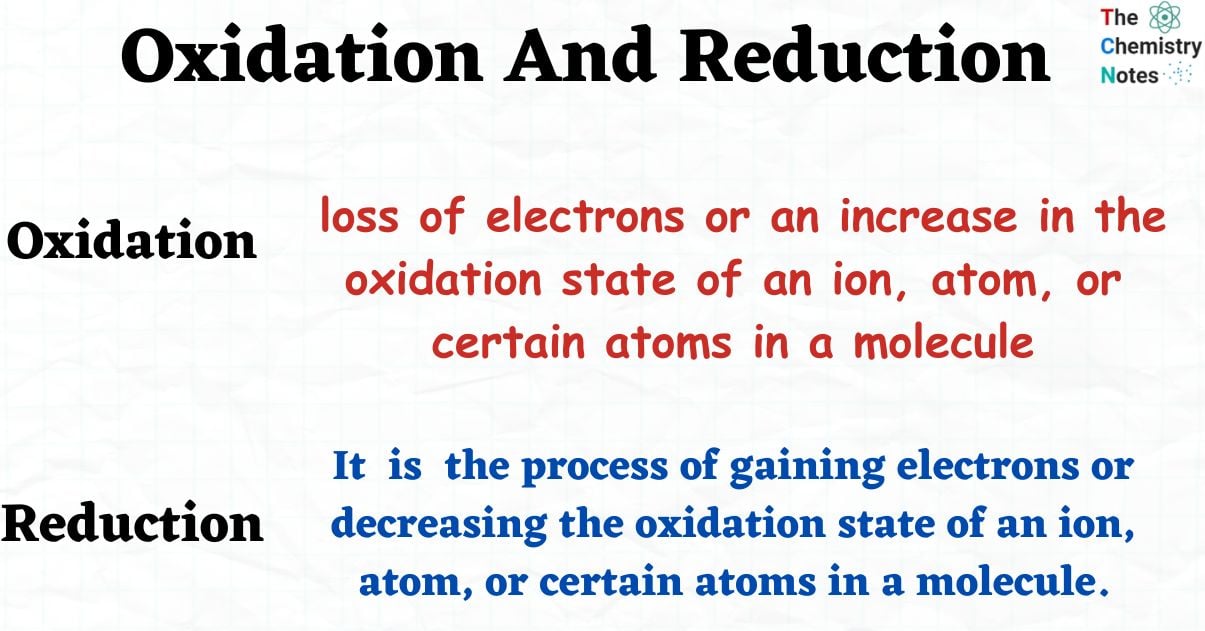
The definition of oxidation is the loss of electrons or an increase in the oxidation state of an ion, atom, or certain atoms in a molecule. A chemical reaction that involves the combination of an element with oxygen is commonly referred to as oxidation.
The chemical reaction between magnesium metal and oxygen results in the formation of magnesium oxide, which is known as the oxidation of magnesium. An oxidizing agent function is to introduce oxygen to another substance.
What is Reduction?
Reduction is the process of gaining electrons or decreasing the oxidation state of an ion, atom, or certain atoms in a molecule.
The origin of the term reduction is from the Latin language, where it means “to lead back”. At a temperature of 2000 Celsius, magnesium oxide reacts with carbon to produce magnesium metal and carbon monoxide, which is an instance of magnesium oxide being reduced to magnesium metal. Reducing agents are responsible for eliminating oxygen from other substances.
Classical Concept Of Oxidation and Reduction
The addition of oxygen or an electronegative component or the removal of hydrogen or an electropositive part is classically referred to as oxidation, and the addition of hydrogen or an electropositive part or the removal of oxygen or an electronegative part is referred to as reduction. It demonstrates that oxidation and reduction are opposing processes.
Two different types of reagents are required to carry out the oxidation-reduction reaction.
Reducing Agents (Reductants): Reduction is a process that involves the addition of hydrogen or any electropositive element or the removal of oxygen or any electronegative element. The reagent that undergoes oxidation is called a reducing agent.
Oxidizing Agents (Oxidants): Oxidation is a process that involves the addition of oxygen or any electronegative element or the removal of hydrogen or any electropositive part. The reagent that undergoes reduction is called an oxidizing agent.
That is, the reducing agent (reductant) and the oxidizing agent (oxidant) are oxidized and reduced, respectively, in the provided oxidation-reduction reaction.
For Example:
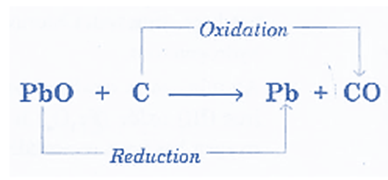
- Reaction of PbO and carbon: During the process, carbon (C) gains oxygen while lead oxide (PbO) loses oxygen. PbO undergoes reduction while C undergoes oxidation.
- Reaction of H2S and Cl2: Here, hydrogen is being removed from hydrogen sulphide (H2S) and is being added to chlorine (C2S). Thus, H2S is oxidised and Cl2 is reduced.

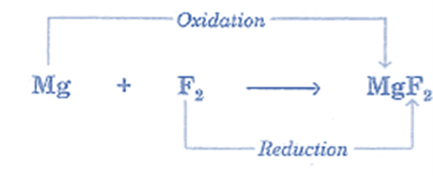
- Reaction between Mg and F2: The electronegative radical fluoride ion (F–) is introduced to magnesium, while the electropositive radical Mg2+ is introduced to fluorine. Consequently, the element Mg undergoes oxidation while the element F2 undergoes reduction.
Electronic Concept of Oxidation and Reduction
The electronic concept of a redox reaction is based on the electron transfer process.
Oxidation: The phenomenon of oxidation involves the loss of one or more electrons by an atom or a group of atoms participating in a chemical reaction. The loss of electrons causes the positive or negative charge of a species to increase or decrease, respectively.
Mg ⟶ Mg+++2e− (loss of electron)
Fe++ ⟶ Fe+++ + e− (increase in positive charge)
2Cl−⟶ Cl2 + 2e−(decrease in negative charge)
Reduction: It is a chemical process whereby an atom or a group of atoms involved in a chemical reaction undergoes a gain of one or more electrons. The gaining of electrons leads to a reduction in the positive charge or an increase in the negative charge of the entity.
O+2e−⟶ O−− (gain of electron)
Fe+++ + e− ⟶ Fe++ (decrease in positive charge)
S + 2e−⟶ S−− (increase in negative charge)
Reducing Agent: It is defined as the reaction by the loss of one or more than one electron. Reducing agent undergoes oxidation, i,e. it always oxidized.
Oxidizing Agent: It is defined as the reagent undergoing reaction by the gain of one or more than one electron. Oxidizing agent undergoes reduction, i.e. it is always reduced.
Oxidation and Reduction in Terms of Oxidation Number
According to this concept, the increase in oxidation number means Oxidation (loss of the electron) whereas the decrease in oxidation number means Reduction (gain of electron).
Zn + H2SO4 ⟶ ZnSO4 + H2
The O.N of Zn increased from 0 to 2 i.e Zn got oxidized.
The O.N of H2 decreased from 2 to 0 i.e H2 got reduced.
The oxidation number (O.N.) of an element is a measure of the residual charge that its atom possesses or seems to possess when all other atoms in the molecule are considered to have been removed as ions, by taking into account the shared electrons with the more electronegative atom.
Half Equations
Half-equations are mathematical expressions that represent one of the two half-reactions involved in a redox reaction.
Redox reactions comprise of two distinct processes, namely oxidation and reduction. Half-equations delineate individual processes in relation to the movement of electrons. One of the equations illustrates the oxidation process, which involves the loss of electrons, while the other equation depicts the reduction process, which entails the acquisition of electrons.
Example:
Ag+ + Al → Ag + Al3+
Reduction half-reaction:
Ag+ + e− → Ag
Aluminum is oxidized, losing three electrons to change from Al to Al3+:
Oxidation half-reaction:
Al → Al3+ + 3e−
To combine these two half reactions and cancel out all the electrons, we need to multiply the silver reduction reaction by 3:
3 (Ag+ + e− → Ag)………………..1)
Al → Al3+ + 3e− ………………..2)
From eqn 1 and 2,
3Ag+ Al → 3Ag + Al3+
- The equation has achieved balance not only in regard to the elements but also with respect to the electrical charge.
- The reactant that has undergone oxidation is referred to as the oxidized substance, Al
- The substance that has undergone reduction is referred to as the reduced reactant, Ag+
- Aluminum serves as both the reducing agent and the oxidized substance.
- The oxidizing agent is the same as the reduced substance: Ag+
References
- Haustein, Catherine Hinga (2014). K. Lee Lerner and Brenda Wilmoth Lerner (eds.). Oxidation–Reduction Reaction. The Gale Encyclopedia of Science (5th ed.). Farmington Hills, MI: Gale Group.
- https://stoplearn.com/classical-concept-of-oxidation-and-reduction/
- Hudlický, Miloš (1990). Oxidations in Organic Chemistry. Washington, D.C.: American Chemical Society. p. 456. ISBN 978-0-8412-1780-5.
- https://www.thoughtco.com/definition-of-oxidation-in-chemistry-605456
- https://chemistrytalk.org/redox-reactions/
- https://unacademy.com/content/neet-ug/study-material/chemistry/concept-of-oxidation-and-reduction/
- https://stoplearn.com/classical-concept-of-oxidation-and-reduction/
