Partition chromatography is a technique primarily employed for the separation of mixture components into two liquid phases: the original solvent and the solvent coating used in the column. It is the most often-used type of liquid chromatography. The chromatographic method known as partition chromatography is unique in that it depends on separating analytes into a mobile phase and a stationary liquid phase. By making use of the varied affinities of the compounds for the two phases, this separation technique produces distinctive bands or peaks in the chromatogram as well as differential migration rates. Partition chromatography is especially flexible and suitable for a wide range of substances because it does not require a solid stationary phase like other chromatographic techniques.

In partition chromatography, a solute’s retention time is determined by how much it passes from the mobile phase to the stationary phase and back again. The polarity of the solutes, the stationary phase, and the mobile phase all influence how much equilibrium partitioning occurs.
In normal-phase partition chromatography, more polar solutes take longer to elute because the polar stationary phase more strongly retains them. The stationary phase is polar and the mobile phase is non-polar, or of low polarity. More polar solutes elute more quickly in reverse-phase partition chromatography because the stationary phase is less effective at retaining them. The stationary phase is non-polar and the mobile phase is polar. Reverse-phase partition chromatography is the more widely used of the two methods.
Interesting Science Videos
What is Partition Chromatography?
- Partition Chromatography involves separating components from two liquid phases: the initial solvent and the solvent film in the column.
- This separation theory was developed in the 1940s and published by Richard Laurence Millington Synge and Archer Martin. It is also known as Liquid-liquid chromatography (LLC). If gas is used as the mobile phase, the process is known as gas-liquid chromatography (GLC).
- By dividing the components between two phases, both of which are present in liquid form, partition chromatography allows the components to be separated from the sample.
- The immiscible solid surface in this process is in the mobile phase when it is covered in the liquid surface in the stationary phase.
- The liquid surface eventually becomes immobile and transforms into a stationary phase as a result of the stationary phase. Shortly after the mobile phase separates from the stationary phase, the components are separated. The variations in partition coefficients are the source of the separation.
- In general terms, the separation is based on a fixed phase and a mobile phase, both of which are liquids.
- When it comes to the components in the mixture, the stationary phase is often an immiscible solvent with low solubility, whereas the mobile phase is a solvent with high solubility. The components of the mixture separate between the two liquids according to their relative affinities for each, which causes the liquids to separate. Numerous fields, including environmental studies and biology, use this method extensively for the effective separation of complex mixtures.
- The solubilities of the components in the stationary and mobile phases differ, which causes the separation process, which results in different migration rates and separation of the components along the stationary phase. Partition chromatography is a potent analytical method that has greatly benefitted the field of chromatography.
- Partition chromatography provides a distinct mechanism for separation from other chromatographic techniques like gas chromatography or liquid chromatography on solid substrates. A wider range of analytes can be successfully separated and a more flexible selection of solvents is made possible by the lack of a solid stationary phase. Due to its versatility, partition chromatography is essential in a wide range of scientific and industrial applications, especially when handling complicated mixtures.
Principle of Partition Chromatography
Partition chromatography operates on the fundamental concept of analytes distributing themselves between two liquid phases that do not mix: stationary and mobile. The partition coefficient, a critical factor in this method, represents the ratio of a solute’s concentration in the stationary phase to its concentration in the mobile phase when equilibrium is reached. This coefficient dictates the extent to which the stationary phase holds onto a specific compound, thus impacting its movement rate and separation within the chromatographic setup.
A dynamic interaction that is essential to the effectiveness of partition chromatography is the equilibrium between the stationary and mobile phases. A balance is reached where each component exists in proportion to its affinity for the stationary and mobile phases while the analytes constantly move between these phases. The position and width of the bands or peaks shown in the chromatogram are determined by this equilibrium, which in turn establishes the foundation for efficient separation.
In summary, the basic principle is described in the following points:
- The technique of separating the components into two steps is how the components are extracted from the sample mixture. Both stages exist in a liquid state.
- The immiscible solid surface that is coated in liquid on the stationary phase of this procedure is in the mobile phase.
- A stationary phase immobilizes the liquid surface, turning it into a stationary phase.
- The components separate as the mobile phase separates from the stationary phase. Various partition coefficients affect the separation.
Partition coefficient in chromatography
The solute gains an equilibrium distribution between the two phases whenever the mobile phase flow is stopped, regardless of the time interval. The partition coefficient in chromatography, denoted as K = CS/CM, provides the concentration in each phase.
Here, CS refers to the solute’s concentration in the stationary phase. The concentration of solute in the mobile phase is referred to as CM.
CS and CM may have distinct units. K = 1 is the case when the solute is split equally between the two phases.
Factors Affecting Partition Chromatography
- Partition chromatography is influenced by several variables, and it is essential to comprehend these parameters to optimize the separation process.
- The selectivity and retention of analytes are influenced by the solvent used in the stationary phase, which is a crucial factor.
- Similar to this, the chromatographic performance is greatly influenced by the solvent’s polarity and viscosity, as well as the composition and characteristics of the mobile phase.
- The effects of temperature also need to be taken into account, since temperature changes can modify the partition coefficients and impact the overall effectiveness of the separation procedure.
Procedure of Partition Chromatography
To effectively separate and analyze the components inside a mixture, partition chromatography requires a set of well-defined processes. These procedures, which are described below, include setting up the stationary phase, loading the sample, choosing the mobile phase, developing the chromatogram, and then identifying and analyzing the separated components.
- Preparation of Stationary Phase
- Preparing the stationary phase is the first stage in the partition chromatography process.
- In partition chromatography, the stationary phase is liquid as opposed to solid in conventional chromatographic methods.
- The efficiency and selectivity of the separation are determined by the stationary phase solvent selection, which is crucial.
- Fluorinated hydrocarbons and silicone oils are examples of frequently used liquids that have a broad range of compatibility with various analytes.
- Loading the Sample
- The sample with the component mixture is placed onto the chromatographic apparatus after the stationary phase is ready.
- It can be accomplished using a variety of methods, including injection, which enables interaction between the sample and the stationary phase to start the separation process.
- As the sample passes through the chromatographic column, components start to be differentially partitioned between the stationary and mobile phases.
- Mobile Phase Selection
- The mobile phase, which is usually a gas or liquid, is essential for allowing the components to pass through the chromatographic system more easily.
- The kind of analytes, the stationary phase being utilized, and the intended separation results all play a role in choosing the right mobile phase.
- The composition and flow rate of the mobile phase affects the resolution and efficiency of the separation process.
- Development of Chromatogram
- The components of the chromatographic column separate as a result of their differential partitioning as the sample moves through the column.
- The chromatogram, a graphical depiction of the elution profile of each component over time, shows this separation.
- The relative abundance and retention periods of each component are revealed in great detail by the chromatogram.
- Detection and Analysis of Separated Components
- Identifying and evaluating each of the separated components is the last stage.
- Several techniques for detection, including mass spectrometry, fluorescence detection, and UV-visible spectroscopy, can be used, depending on the type of analytes.
- Following data collection, analysis is performed to ascertain the identification, amount, and purity of each separated component, yielding important information about the original mixture’s composition.
Types of Partition Chromatography
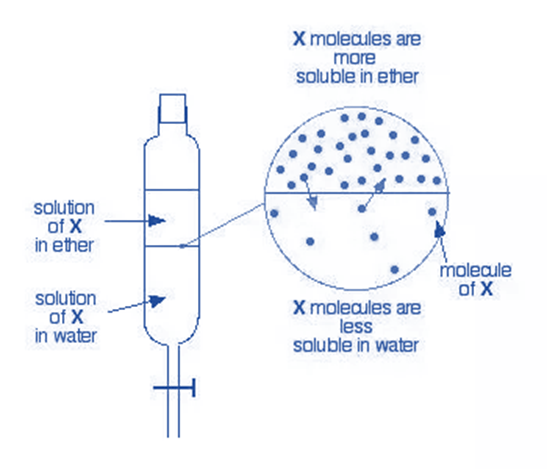
Liquid-Liquid Chromatography
Partition chromatography in the form of liquid-liquid chromatography separates mixture components based on how soluble they are in the stationary and mobile phases, two immiscible liquids. It is a useful method for sorting and cleaning materials with different polarity.
A liquid that is miscible with the mobile phase—typically a solvent—makes up the stationary phase in this procedure. After being put on top of the stationary phase, the sample mixture is allowed to separate into the two liquids. The mobile phase is then passed over the stationary phase, and the components are constantly eluted according to their solubility in the two liquids.
- Paper Chromatography:
- Paper chromatography is a simple, low-cost technique for the separation and analysis of mixture components.
- It is predicated on the principle of partition chromatography, which divides components into two groups: a mobile phase, represented by a solvent, and a stationary phase, represented by a sheet of filter paper.
- The sample mixture is put on top of the paper and allowed to diffuse into the fibers once the paper fibers have become soluble in the solvent. The solvent is drawn up the paper by capillary action, causing the constituents to separate based on their solubility and partition coefficients. Consequently, the mixture is separated onto the paper into many spots or bands that each represent different components.
- Column Chromatography:
- Column chromatography is a type of partition chromatography in which mixture components are separated based on how efficiently they partition between a stationary and mobile phase.
- The stationary phase is contained in a column that the mixture passes through. When the mobile phase—typically a solvent—is passed through the column, the components of the mixture separate into the stationary and mobile phases based on their partition coefficients.
- The combination then divides into discrete peaks on the chromatogram as the separated components elute from the column at different times.
- Thin-layer Chromatography:
- Partition chromatography includes thin-layer chromatography (TLC).
- A thin layer of adsorbent on a plate represents the stationary phase, and a solvent represents the mobile phase.
- The sample combination is applied to the thin layer, and the solvent is drawn up the plate by capillary action.
- The components are then divided between the stationary and mobile phases according to their corresponding partition coefficients, causing the mixture to separate into discrete spots or bands on the plate.
Gas-Liquid Chromatography
A liquid stationary phase is usually immobilized on a solid support, and a gaseous mobile phase is used in gas-liquid chromatography (GLC). For volatile and thermally stable substances, such as organic molecules and gasses, this technique is frequently used. GLC is excellent in separating substances according to their affinities for the liquid stationary phase and vapor pressures, allowing for sensitive and accurate analysis. When working with non-volatile or thermally labile substances, GLC could be limited.
Application of Partition Chromatography
- Pharmaceutical Industry: In pharmaceutical analysis, partition chromatography is essential, especially for drug development and discovery. It helps in the separation and analysis of different ingredients in medication compositions, guaranteeing efficacy and quality control.
- Environment Monitoring: In environmental science, partition chromatography is utilized to detect and quantify pollutants, such as pesticides, herbicides, and heavy metals, in soil, water, and air samples. This technique aids in monitoring environmental pollution levels and assessing potential risks to ecosystems and human health.
- Forensic science: Forensic labs use partition chromatography to examine intricate concoctions of substances discovered at crime scenes. It helps with the identification and concentration measurements of illegal substances, including explosives and drugs, which are essential for legal investigations.
- Food and Beverage Industry: In the food and beverage sector, partition chromatography is used to test and quantify different food product components, including flavors, additives, and pollutants. This promotes quality assurance, food safety, and adherence to legal requirements.
- Biochemistry Analysis: In biochemistry and biotechnology, partition chromatography is a commonly employed technique for the extraction of biomolecules like as proteins, nucleic acids, and carbohydrates from intricate biological specimens. It makes it easier to isolate and characterize particular molecules, which is necessary for both industrial and research purposes.
- Clinical diagnosis: In clinical laboratories, partition chromatography is utilized for analyzing biological fluids, such as blood, urine, and saliva, to diagnose diseases and monitor patients’ health. It enables the separation and quantification of biomarkers and metabolites, aiding in disease detection and treatment monitoring.
- Chemical Process Industry: In the chemical process industry, partition chromatography is used to separate and purify chemical components during production processes. Enhancing process efficiency and product quality helps produce pharmaceutical intermediates and high-purity chemicals.
Advantages of Partition Chromatography
- One advantage of partition chromatography is that it is an inexpensive and straightforward separation technique.
- The partition chromatography method can be used to isolate both organic and inorganic substances.
- Time is saved since this procedure produces accurate findings, is highly effective, and separates compounds quickly.
- Partition chromatography offers better selectivity since it is simple to alter the mobile phase.
Disadvantages of Partition Chromatography
- Sometimes the high volume of the mobile phase is required for separation.
- Data storage is limited in several partition chromatography applications.
- Automation is making it more difficult and costly.
- Gas-liquid chromatography can differentiate only volatile compounds, and in sample combinations, it can separate only the soluble analytes.
References
- https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Instrumental_Analysis_(LibreTexts)/28%3A_High-Performance_Liquid_Chromatography/28.04%3A_Partition_Chromatography
- https://www.chemistrylearner.com/chromatography/partition-chromatography
- https://byjus.com/chemistry/partition-chromatography/
- https://testbook.com/chemistry/partition-chromatography
- https://lab-training.com/partition-chromatography/
- https://collegedunia.com/exams/partition-chromatography-fundamentals-procedure-types-questions-chemistry-articleid-2618
- https://www.onlinebiologynotes.com/partition-chromatography-principle-instrumentation-and-application/
- https://infinitylearn.com/surge/chemistry/partition-chromatography/
