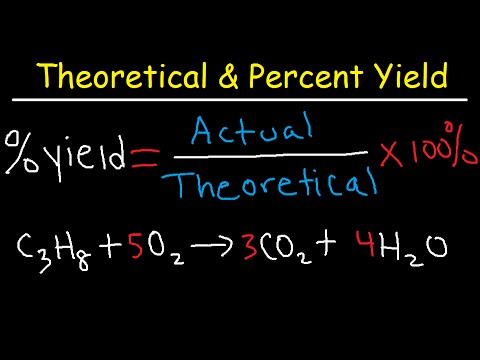
The percentage that represents the difference between the theoretical and actual yields is called the percent yield. In chemistry, yield is the amount of product produced by a reaction relative to the amount of reactant consumed; it is typically given as a percentage. The percentage yield is the difference between the amount of product that was actually produced and the maximum yield that was calculated.
Interesting Science Videos
What is Percent Yield?
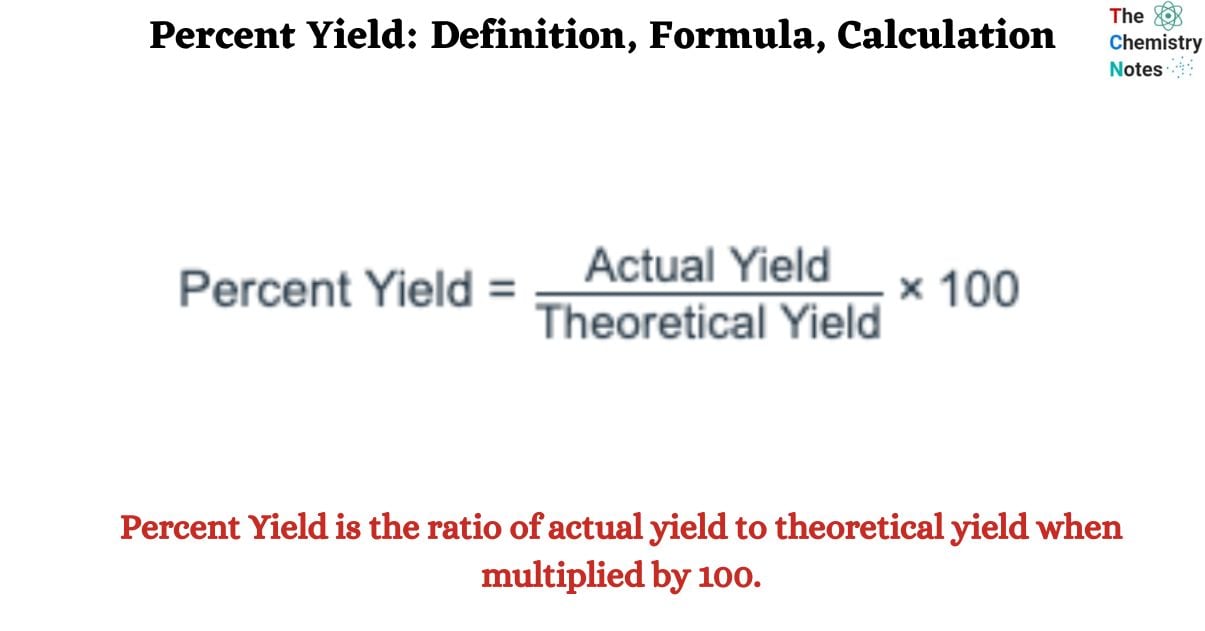
Percent Yield is the ratio of actual yield to theoretical yield when multiplied by 100. Analyzing the percentage of reactant conversion to product is crucial in chemistry. The stoichiometric equation, which represents the chemical reaction, is used to compute the theoretical yield. After the reaction is finished, the actual yield is measured in the laboratory. Note that the theoretical yield and actual yield should have the same units. When expressing theoretical and actual yield, the mole or gram is usually the favored unit of measurement.
Percent Yield Formula
The percent yield formula is actual yield divided by theoretical yield in moles multiplied by 100%:
Percent Yield = Actual Yield/Theoretical Yield x 100%
It makes no difference whether you express actual and theoretical yield in grams or moles, as long as you use the same units for both values.
How to Calculate Percent Yield?
The real yield and the theoretical yield are the two values needed to calculate the percent yield. The mole ratio of reactants to products determines yield. The amount of product that is actually obtained from a reaction or experiment is known as the actual yield. After the product is weighed, you convert the mass—which is typically measured in grams—to moles.
The concept of stoichiometry yields the theoretical yield. That is, it originates from the balanced equation for the chemical reaction based on the mole ratio of reactants to products. The next step is to identify the limiting reactant when you have the balanced equation. Since the limiting reactant is used up before the other reactant runs out, it is the reactant that limits the amount of product. The limiting reactant in a decomposition reaction is the one that may exist exclusively in the reaction. You compare the molar masses and mole ratios in different reactions. The theoretical yield is then computed using the mole ratio and the number of moles of the limiting reactant. Lastly, calculate out the potential yield.
- The chemical equation for the reaction must be balanced. Take note of the number of moles of reactants and products.
- Determine the limiting reactant. For each reactant, determine its molar mass. Determine the total number of moles in each species.
- Find out which reactant restricts the reaction by using the mole ratio.
- Calculate the theoretical yield using the mole ratio and the number of moles of the limiting reactant.
- Measure the product’s weight. This is the actual yield.
- Check that the actual and theoretical yields have the same units (grams or moles).
Using the actual and theoretical yields, compute the yield percentage.
Percent Yield Example
Using an example, let us comprehend the idea of Percent Yield.
Take the decomposition of calcium carbonate as an example.
CaCO3 → CaO + CO2
According to the reaction shown above, only one mole of the product CaO is produced for every mole of the reactant CaCO3. This indicates unequivocally that the mole ratio of the reactant to product is 1:1.
Assume we have two moles of reactant for this reaction. Thus, we will also receive two moles of the product. However, keep in mind that the yield was actually 1.5 moles. We determine the ratio between the actual and projected yields in order to get the percentage yield.
So, the percentage yield becomes,
P = (1.5/2) × 100 % ⇒ P is 75%
Why Percent Yield is always less that 100?
Percent yield is always less than 100% (typically by a significant margin), however a value greater than 100% can be calculated.
There are several reasons why percent yield is always low.
- Not all reactions are completed.
- Reactions can also occur in reverse when reactants and products are in equilibrium.
- Simultaneous reactions take place, resulting in the conversion of some reactant into one or more side products.
- Other species or contaminants could be interfering with the process.
- Product evaporates while being transferred.
- Purification results in product loss.
Even yet, sometimes we get more product than you expected. Sometimes the formation of a product is aided by an impurity. However, the actual yield is typically higher. But if an impure product is collected, the mass exceeds the predicted yield. The most common issue is weighing a product that isn’t totally dry. Solvent makes up a portion of the mass, so it looks like you obtained more product than you expected.
Video on Percent Yield
References
- https://www.wikihow.com/Calculate-Percent-Yield-in-Chemistry
- https://www.cuemath.com/percentage-yield-formula/
- Whitten, Kenneth W.; Davis, Raymond E; Peck, M. Larry (2002). General Chemistry. Fort Worth: Thomson Learning. ISBN 978-0-03-021017-4.
- https://sciencing.com/how-to-calculate-percent-yield-13710472.html
- Vogel, Arthur Israel; Furniss, B. S; Tatchell, Austin Robert (1978). Vogel’s Textbook of Practical Organic Chemistry. New York: Longman. ISBN 978-0-582-44250-4.
- https://www.geeksforgeeks.org/percent-yield-formula/