The Perkin condensation reaction, also known as the Perkin reaction, is an organic reaction used to produce, unsaturated aromatic acid via condensation of an aromatic aldehyde and an acid anhydride in the presence of an alkali salt of the acid serving as a weak base. In general, this type of reaction is only relevant to aromatic aldehydes and is beneficial for producing substituted cinnamic acid. The Perkin reaction has a major application in the laboratory synthesis of the phytoestrogenic stilbene resveratrol. This reaction occurs when aromatic aldehydes react with alkanoic anhydrides in the presence of alkanoate.
The Perkin reaction is named after the English chemist William Henry Perkin. Perkin provided the first example of a condensation reaction of this type in 1968, involving the production of coumarin by condensing the sodium or potassium salt of salicylaldehyde with acetic anhydride. In general, such a reaction is only relevant to aromatic aldehydes and is beneficial for preparing substituted cinnamic acid.
Interesting Science Videos
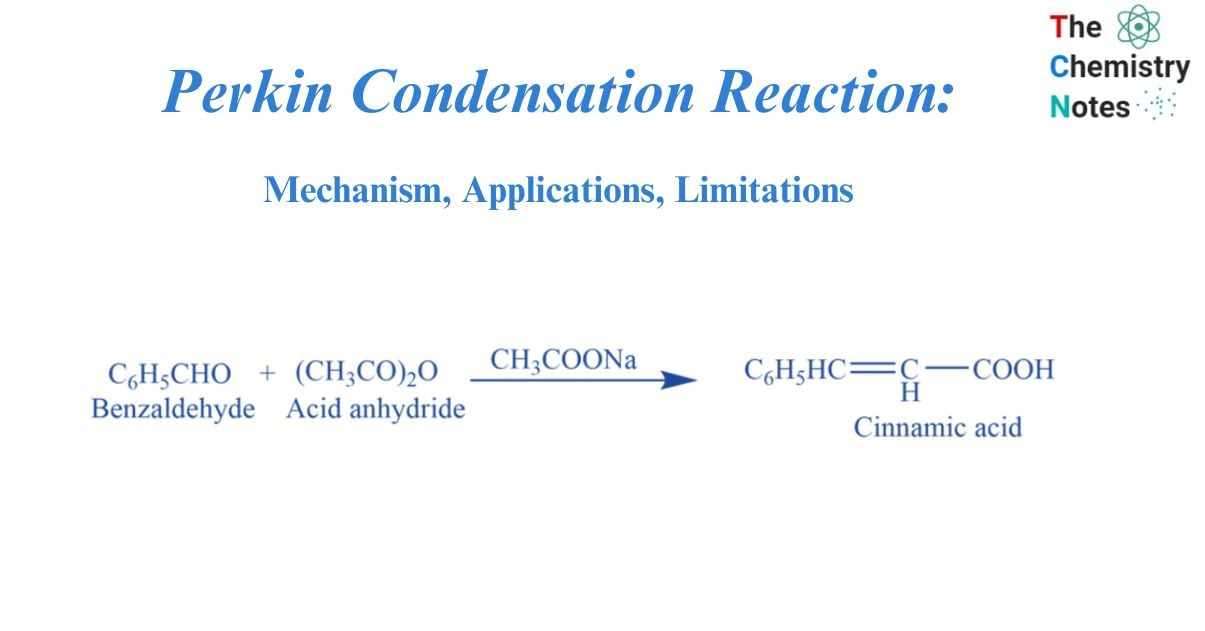
What is Perkin Condensation Reaction?
The condensation reaction of benzaldehyde with an acid anhydride in the presence of alkali salt of the acid gives alpha, beta-unsaturated acid i.e., Cinnamic acid. This reaction is called Perkin’s condensation reaction.

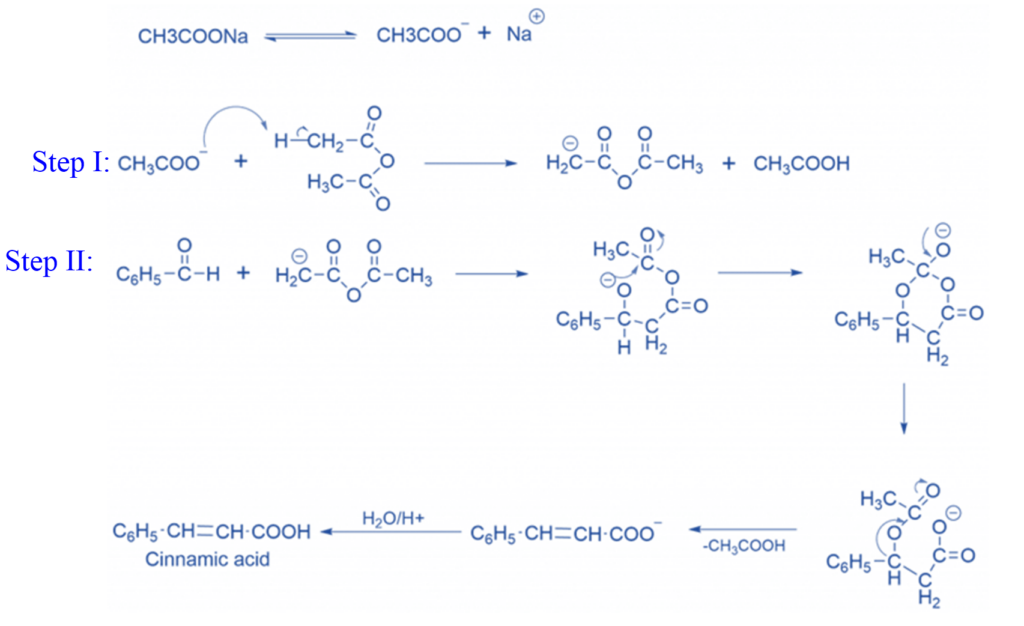
Mechanism of Perkin Condensation Reaction
Step 1: The anhydride’s carbon contains acidic protons. These carbons are known as active methylated carbons. When the base removes the α-hydrogen atom, the carbanion intermediate is created.
Step 2: Anhydride nucleophilically attacks a carbonyl molecule. The electrophilic carbonyl carbon of the carbonyl molecule frequently interacts with nucleophilic deprotonated anhydride to produce a 6-membered intermediate. The ring opening creates a carboxylate ion. Which on hydrolysis produces α,β-unsaturated carboxylic acid.
Applications ofPerkin Condensation Reaction
- The Perkin reaction is most commonly employed to produce aryl-substituted acrylic acids, and catalytic hydrogenation can yield beta-aryl derivatives of saturated carboxylic acids. This process is also used to make several commercially relevant compounds:
- Furylacrylic acid is produced from furfural with a yield of 65-70%.
- Coumaric acid can be produced by dehydrating salicylaldehyde.
- The phytoestrogenic stilbene resveratrol is synthesized by this reaction method
- Perkin Condensation is used in laboratories to produce cinnamic acid. Cinnamic acids are fragrant unsaturated carboxylic acids found in cinnamon and shea butter.
- It is used to make alpha and beta-unsaturated aromatic acids, which are important in the pharmaceutical industry.
- The aromatic aldehydes such as pthalaldehyde, isophthalaldehyde, and terepthaldehyde also undergo this condensation process, yielding benzenediacrylic acid in 28-80% yields.
Limitations of Perkin Condensation Reaction
- In general, this type of reaction is only applicable to aromatic aldehydes, and the condensation involves only the anhydride’s α-hydrogen atoms.
References
- Morrison R. T. & Boyd R. N. (1983). Organic chemistry (4th ed.). Allyn and Bacon.
- Smith M. & March J. (2001). March’s Advanced Organic Chemistry: Reactions Mechanisms and Structure (5th ed.). Wiley.
- Ghosh, S.K., Advanced General Organic Chemistry, Second Edition, New Central Book Agency Pvt. Ltd., Kolkatta, 2007.
- Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and Company Ltd, New Delhi, 1992. production of polyesters, polyurethanes, and alkaline resins.

Can we take any aldehyde as a reagent?