One of the most commonly used acid-base indicators for determining the endpoint in acid-base titrations is phenolphthalein. In some laxatives, it serves as the active component. However, due to the substance’s alleged carcinogenicity, phenolphthalein is no longer used as a laxative.
Interesting Science Videos
What is Phenolphthalein?
Phenolphthalein is an organic compound that serves as a laboratory reagent and pH indicator. It stimulates the intestinal mucosa and constricts smooth muscles, causing laxative effects. The fact that phenolphthalein may be carcinogenic, however, has led to its discontinuation as a laxative.
A few drops of the appropriate indicator added to an unknown solution can reveal its pH value. A pH indicator added to the unknown solution changes color to indicate the neutral point. It is a colorless, weak acid that is widely used in titration experiments to indicate the titration endpoint. The formation of pink color indicates the endpoint. The phenolphthalein indicator turns light pink under normal alkaline conditions and colorless under acidic and extremely alkaline conditions.
History and Synthesis of Phenolphthalein
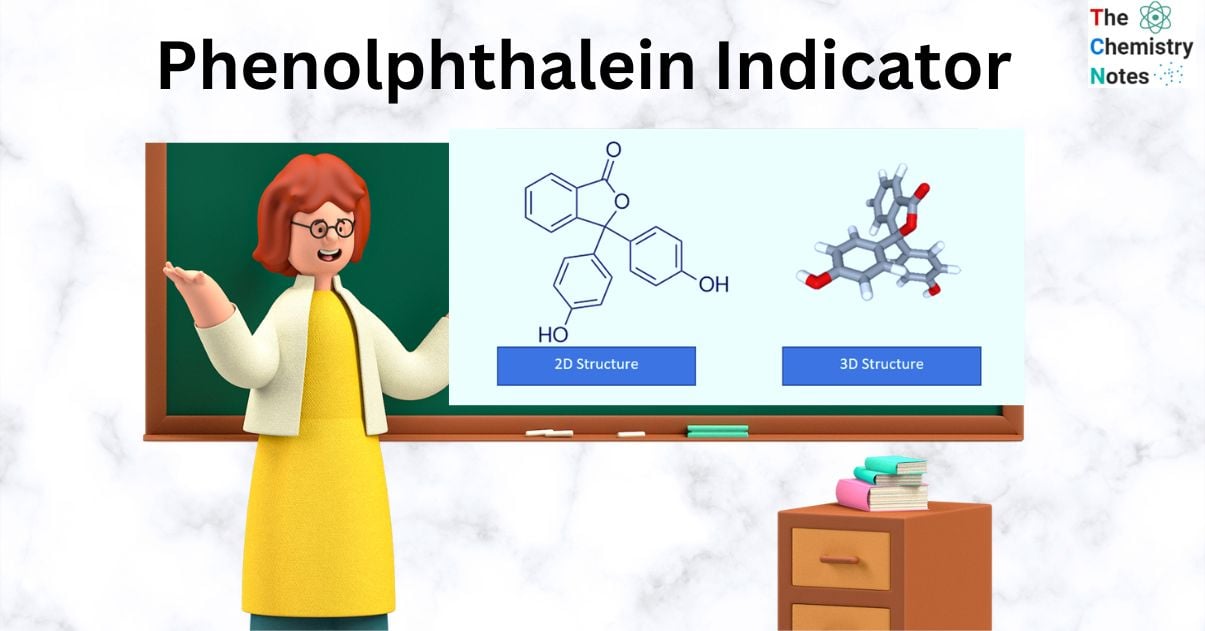
In 1871, Adolf von Baeyer, a German chemist, discovered phenolphthalein. He produced it by fusing phenol and phthalic anhydride in the presence of sulfuric acid or zinc chloride. Triphenylmethane dyes and phenolphthalein have a close resemblance.
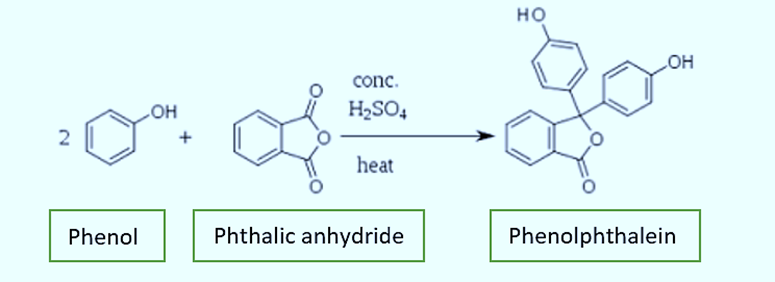
Structure of Phenolphthalein
With three hexagonal structures, one pentagonal structure, two alcoholic groups, and one ketone group, the compound with the formula C20H14O4 is the structure of phenolphthalein. Additionally, the carbon, hydrogen, and oxygen chains that makeup phenolphthalein’s structure. Phenolphthalein is commonly abbreviated as “HIn”, “HPh”, “phph”, or simply “Ph”.
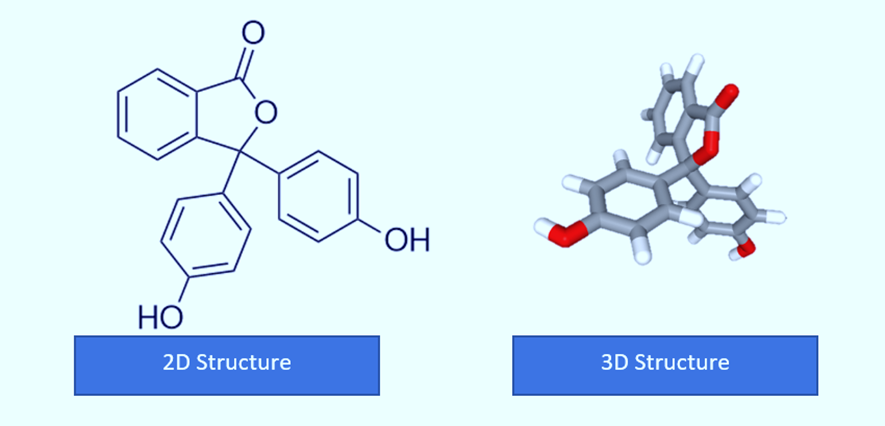
The HPh derivative, which at various pH levels absorbs at visible light wavelengths, undergoes conjugation of its electrons, which results in color change. It is due to the lactone ring’s different open and closed structures in these compounds.
The pH Range
A pH of 7 is considered neutral on the pH scale, which ranges from 0 to 14. A substance is said to be acidic if its pH is less than 7, and basic if its pH is greater than 7.
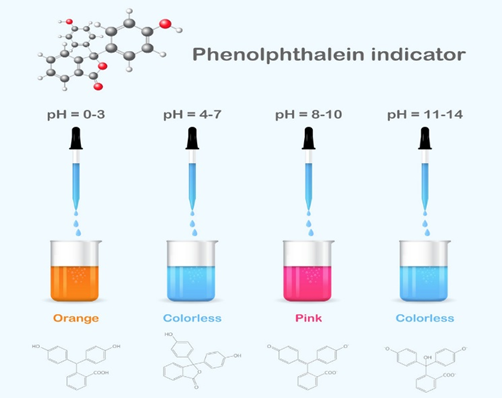
In alkaline solutions, HPh, which is inherently colorless, turns pink. The substance doesn’t change color throughout the range of acidic pH levels, but at a pH of 8.2, it starts to turn pink and then bright magenta at pH 10 and above.
- It protonated to form an orange color in a strongly acidic solution (pH = 0–3).
- It is colorless between extremely acidic and mildly basic conditions (pH = 4–7).
- The most common pink dianion form is produced when an alkali is present (pH = 8–10).
- the HPh solution is colorless in the presence of strong alkali (pH range 11–14).
Properties
- In its crystalline form, HPh is white-yellow.
- It comes in the form of a white, crystalline powder that dissolves in water. According to the pH scale range of the aqueous solution, it comes in four different forms.
- It dissolves easily in alcohol and only slightly in water.
- It is carcinogenic and has no taste or smell.
| PROPERTY | VALUE |
| Melting Point | 262.5 °C |
| water solubility | 400 mg/L |
| logP | 2.41 |
| pKa | 9.7 (at 25 °C) |
| Appearance | White crystalline powder |
| Density | 1.277 g/cm3 at 32 °C |
| Molar mass | 318.328 g mol-1 |
Application/ Uses
- It is frequently employed in acid-base titrations as an indicator.
- It is a universal indicator like methyl red, bromothymol blue, and thymol blue.
- Although it can be used as a laxative, it is not advised because this substance may be carcinogenic.
- It is employed to test the carbonation of cement. When HPh is applied to cement that is carbonating, it stays colorless (pH 8.5–9) while when it is applied to normal cement, it turns pink.
- In toys, it is used as a component of disappearing inks or dye. In our disappearing inks, HPh and sodium hydroxide were combined. The pink color vanishes when it reacts with the carbon dioxide in the atmosphere.
- The Kastle-Meyer test is used to identify particular substances in blood using a reduced form of HPh dye.
Preparation of Phenolphthalein Indicator
In comparison to ethanol or ether, HPh is only slightly soluble in water (400 mg/L). In general, you either dissolve the powdered dye in 50% alcohol or first dissolve it in alcohol and then dilute it with water.
Here’s how to make a 1% HPh indicator solution:
- Weigh one gram of HPh
- It should be dissolved in 100 milliliters of water and 50% ethanol. As an alternative, disperse it in a mixture of 50 milliliters of absolute ethanol and 50 milliliters of water.
Here’s how to make a 0.5% HPh indicator solution:
- Weigh out 0.5 grams of HPh.
- Add 50 milliliters of ethanol to it to dissolve it.
- To reach a final volume of 100 milliliters, add distilled water.
However, you also can buy a pre-mixed HPh indicator solution;
- https://www.amazon.com/Phenolphthalein-Indicator-Solution-Dropper-containing/dp/B01M07V5LA
- https://www.sciencecompany.com/ HPh-pH-Indicator-1-oz-P6364
- https://www.wardsci.com/store/product/8883525/ HPh
References
- Wittke, Georg (1 March 1983). “Reactions of phenolphthalein at various pH values”. Journal of Chemical Education.
- Kunimoto, Ko-Ki (February 2001). “Molecular structure and vibrational spectra of phenolphthalein and its dianion”
- https://www.priyamstudycentre.com/2021/11/phenolphthalein-indicator.html
- https://www.chemistry-online.com/lab/experiments/synthesis-of-phenolphthalein/
