The modern periodic table, created by Dmitri Mendeleev, listed elements in ascending atomic mass order. Groups of elements with similar physical and chemical properties constitute the columns.
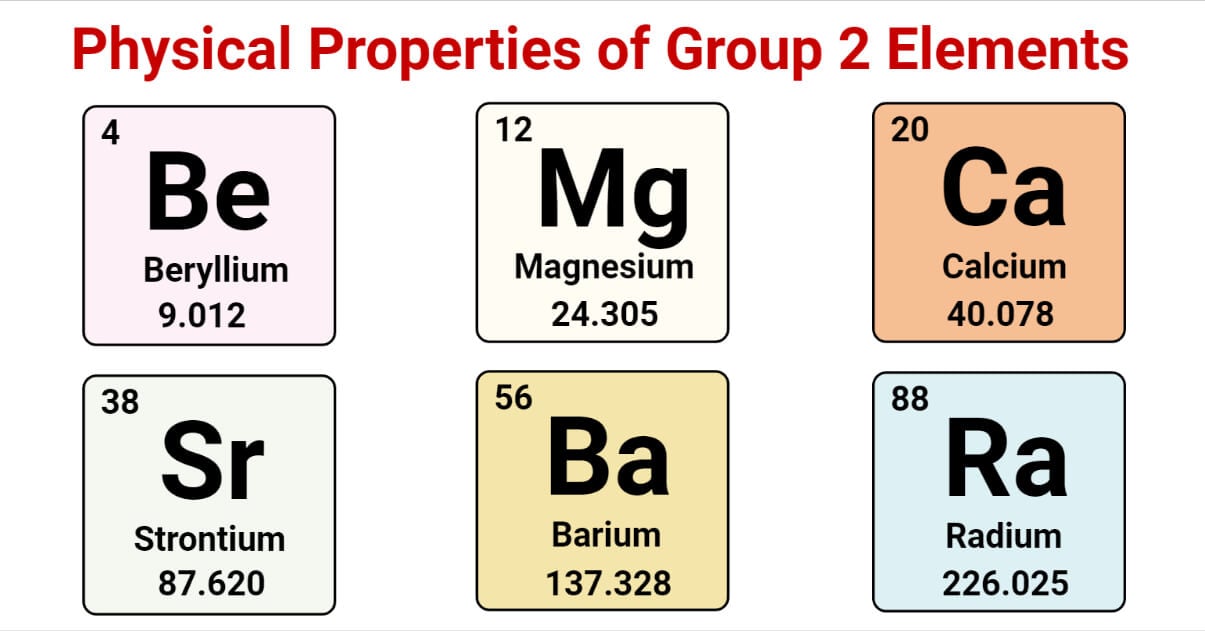
The elements in Group 2 have atoms whose electronic configurations end with two electrons in their outermost principal quantum shell, and those two outer electrons are positioned in an s subshell. The electronic configurations of the first five elements in Group 2 continue to follow:
| Elements in Group II | Symbol | Electronic Configuration |
| Beryllium | Be | 1s22s2 |
| Magnesium | Mg | 1s22s22p63s2 |
| Calcium | Ca | 1s22s22p63s23p64s2 |
| Strontium | Sr | 1s22s22p63s23p63d104s24p65s2 |
| Barium | Ba | 1s22s22p63s23p63d104s24p64d105s25p66s2 |
| Radium | Ra | 1s22s22p63s23p63d104s24p64d105s25p64f145d106s26p67s2 |
When alkaline metals are mixed with water, they produce an alkaline solution. Because their oxides do not dissolve in water and do not heat, they are referred to as earth metals. In the early centuries, scientists used the term earth to refer to any material that possessed these two properties. Since all group 2 elements’ oxides are insoluble in water and heat resistant. As a result, group 2 elements are known as alkaline earth metals.
Interesting Science Videos
Group 2 Elements Physical Properties
An element’s physical properties are those that can be determined without changing the element in any way. However, there are no chemical reactions required to determine the properties of an element or its atom. Elements in the same group on a periodic table have similar physical properties.
Atomic Number and Atomic Mass of Group 2 Elements
| Elements | Be | Mg | Ca | Sr | Ba | Ra |
| Atomic Number (Z) | 4 | 12 | 20 | 38 | 56 | 88 |
| Atomic Mass | 9.012 | 24.31 | 40.08 | 87.62 | 137.3 | 226.0 |
Atomic Radii of Group 2 Elements
The atomic radius is the distance between an atom’s nucleus and its outermost electrons. However, this is only a cursory understanding because the border of the electron cloud of atoms lacks a definite edge.
| Elements | Be | Mg | Ca | Sr | Ba | Ra |
| Atomic Radius (pm) | 105 | 150 | 180 | 200 | 215 | 215 |
- Atomic Radius increases as we move down the column.
- They have a smaller radius than corresponding alkali metals and are larger than other atoms of the same period.
Ionic Radius of Group 2 Elements
Even though ionic radii are difficult to quantify with any certainty and vary depending on the ion’s environment, it matters, for example, what the ion’s coordination is (how many oppositely charged ions are touching it) and what those ions are. However, it follows that if you want to make reliable comparisons with ionic radii, they must come from the same source.
In the case of Group 2 it follows the following trend:
RBe ˂ RMg ˂ RCa ˂ RSr ˂ RBa
- Ionic radius also increases down the periodic table column because of the addition of an electron to the same energy level.
- Both s-electrons can be lost in alkaline earth elements, therefore causing them to be doubly positive cationic.
- A cationic atom’s radius is smaller than that of a neutral atom.
RBe2+ ˂ RMg2+ ˂ RCa2+ ˂ RSr2+ ˂ RBa2+
| Elements | Be | Mg | Ca | Sr | Ba | Ra |
| Ionic Radius (pm) | 34 | 78 | 106 | 127 | 143 | 152 |
Density of Group 2 Elements
The volume of the atoms is reduced because their radius is smaller. Furthermore, due to the presence of two valence electrons, atoms have stronger metallic bonding. As a result, alkaline earth metals are denser and more sturdy than alkali metals. From magnesium to radium, the density of alkaline earth metals generally increases, with calcium having the lowest density.
| Elements | Be | Mg | Ca | Sr | Ba | Ra |
| Density (g/cm3) | 1.85 | 1.74 | 1.55 | 2.63 | 3.65 | 5.50 |
Ionization Energy of Group 2 Elements
The energy required to remove an electron from a gaseous atom or ion is known as ionization energy. Also, it can be considered as a measure of the difficulty of removing an electron or the strength with which an electron is bound. The more difficult it is to remove an electron once the ionization energy increases. As a result, ionization energy is a reactivity indicator. Ionization energy is significant because it can be used to predict chemical bond strength.
Alkaline earth elements can contribute both valence electrons to form an octet noble gas configuration. As a result, they have two ionization energies.
| Elements | Be | Mg | Ca | Sr | Ba | Ra |
| Ionization Energy ( KJ/mol) | 899.5 | 737.7 | 569.8 | 549.5 | 502.9 | 509.3 |
Thus, their ionization energies decrease as they move down the group. Ra, on the other hand, has slightly higher ionization energy than Ba.
First Ionization Energy
The first ionization energy of metals is the amount of energy required to remove the first electron from a neutral atom.
X(g)⟶X(g)+ + e−
In the case of alkali earth metals, it is bigger than an alkali metal atom because of the smaller radii and the electrons being closely bound by the higher nuclear charge, and electrons are removed from a fully filled and thus stable subshell.
Second Ionization Energy
In alkaline earth metals, the second ionisation energy required for the second electron from the cation is greater than the atom’s first ionisation energy but less than the second ionisation energy of any alkali metal.
Despite the higher ionization energy, the atom can remove both electrons because it assumes a noble gas configuration, and the smaller size and higher charge help overcome the higher ionization energy by generating higher lattice energy due to the close packing of atoms or ions in solids. Liquids also have higher hydration energy due to their higher solvation.
IE Be>IEMg>IECa>IESr>IEBa>IERa
Things to be noted:
- The small beryllium atom will require the most ionization energy to remove the valence electron.
- The valence electron becomes shielded by the inner electrons as the atomic size increases, hence, making it easier to remove with less energy. As a result, as the atomic number or size increases, so does the ionization energy.
- On the other hand the alkaline earth elements in Group 2 are all divalent electropositive metals with a fixed oxidation state of 2.
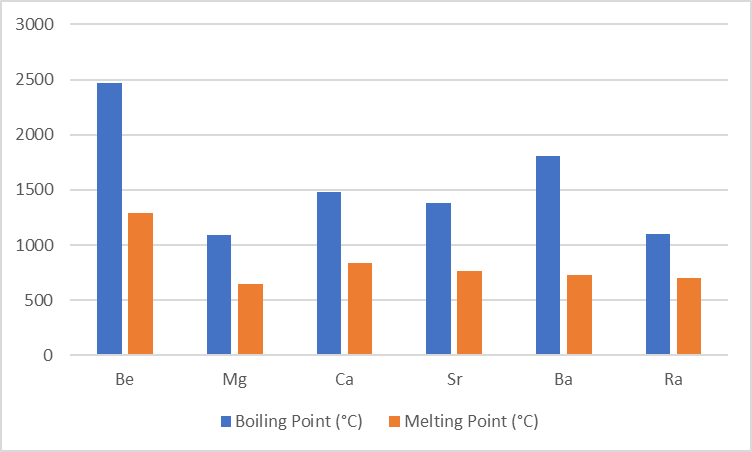
Melting and Boiling Points of Group 2 Elements
Comparatively, Alkaline earth metals have higher melting and boiling points than alkali metals. This is due to their smaller size and strong metallic bonding in a close-packed structure.
| Elements | Be | Mg | Ca | Sr | Ba | Ra |
| Boiling Point (°C) | 2471 | 1090 | 1484 | 1384 | 1805 | About 1100-1700 |
| Melting Point (°C) | 1287 | 650 | 842 | 769 | 727 | About 700 |
Flame Coloration of Group 2 Elements
Alkaline earth metal salts, like alkali metal salts, impart a distinct color to the flame. Certainly, the energy required for an electronic transition between available energy levels in alkaline earth metals falls in the visible spectrum region. When alkaline earth elements and their compounds are heated, electrons absorb energy and are excited to higher levels. When they return to their initial state, the absorbed energy is emitted as visible light of a specific wavelength.
| Elements | Flame Colour |
| Calcium | Brick Red |
| Strontium | Crimson Red |
| Barium | Apple Green |
| Radium | Crimson |
Except for beryllium and magnesium, heating produces a characteristic color to the flame that is reflective of their emission or absorption spectrum and can be used to identify them. The nucleus strongly holds the electrons of beryllium and magnesium atoms because they are smaller in size. Meanwhile, they require a significant amount of energy to excite electrons to higher energy levels, which is not available in the bunsen flame. As a result, they do not color the flame.
Nature of Bonds Formed
Alkaline earth metals, like alkali metals, form ionic compounds that are less ionic than alkali metal compounds. The tendency to form ionic compounds increases as one moves down the group. They form ionic compounds due to their low ionization enthalpies. Because their ionization enthalpies are higher than those of the corresponding alkali metals, their compounds are less ionic. As the ionization enthalpy decreases, consequently the tendency to form ionic compounds increases down the group.
However, Be forms a covalent bond because of its smaller size and high ionization enthalpy.
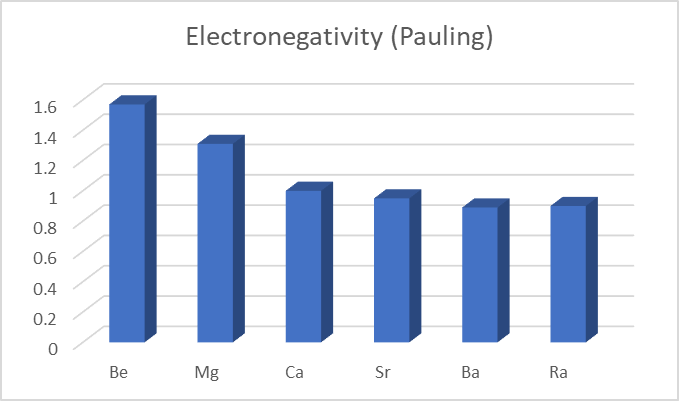
Electronegativity
The ability of an atom to attract a bonding pair of electrons is called electronegativity.
Firstly, as you move down the periodic table, electronegativity decreases. Meanwhile we all know, atomic radius increases as one moves down the group. This means that any bonded electrons are further away from the nucleus, and thus their attraction is weaker.
Beryllium, on the other hand, has a high electronegativity and does not want to lose its electrons. Instead, it clings to them and shares them in a covalent bond with chlorine. Because of this, beryllium forms covalent molecules rather than ionic compounds.
| Elements | Be | Mg | Ca | Sr | Ba | Ra |
| Electronegativity (Pauling) | 1.57 | 1.31 | 1 | 0.95 | 0.89 | 0.9 |
Hydration Enthalpy
Hydration enthalpy is the enthalpy change that occurs when one mole of a gaseous ion dissolves in enough water to form an infinitely dilute solution under standard conditions of 1 bar pressure.
Hydration enthalpy, also known as hydration energy, is always negative. Hence, the trend in the group 2 hydration enthalpy is: Be2+>Mg2+>Ca2+>Ba2+>Ra2+
| Elements | Be | Mg | Ca | Sr | Ba |
| Hydration Enthalpy (KJ/mol) | -506 | -406 | -330 | -310 | -276 |
Because alkaline earth metal ions are smaller in size than alkali metal ions, their hydration enthalpies are greater. Alkaline earth metal compounds are more extensively hydrated than alkali metal compounds.
Solubility of Group 2 Elements
Group 2 elements are insoluble in water compared to other metals. However, the solubility of their compounds varies. Smaller ions have a higher charge density and, therefore, can be dissolved by a greater number of water molecules. As a result, this increases the hydration enthalpy and makes the hydrated ions more stable.
Solubility of Be2+ > Solubility of Mg2+ > Solubility of Ca2+ > Solubility of Sr2+ > Solubility of Ba2+
Oxidation States
The alkaline earth metals have two more electrons than the nearest noble gas configuration. As a result, they can easily lose these two electrons, forming a divalent cation. In conclusion, the oxidation state of alkaline earth metals is always +2.
Color of Element
| Element | Be | Mg | Ca | Sr | Ba | Ra |
| Colour | Grey | Silvery white | Silvery white | Silvery white | Silvery white | White |
Have a look at the video for the summary of the properties of Alkaline earth metals.
References
- Smith, D. (1990). Inorganic Substances: A Prelude to the Study of Descriptive Inorganic Chemistry (Cambridge Texts in Chemistry and Biochemistry). Cambridge: Cambridge University Press. doi:10.1017/CBO9780511622922
- Lee, J D. Concise Inorganic Chemistry. London: Blackwell Science, 2006. Print.
- Cotton, F A, and F A. Cotton. Advanced Inorganic Chemistry. , 1999. Print.
- Mingos, D. M. P. Essential Trends in Inorganic Chemistry. Oxford University Press, 1998.
- https://byjus.com/jee/alkaline-earth-metals/
- https://www.britannica.com/science/alkaline-earth-metal/Physical-and-chemical-behaviour
- https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Supplemental_Modules_and_Websites_(Inorganic_Chemistry)/Descriptive_Chemistry/Elements_Organized_by_Block/1_sBlock_Elements/Group__2_Elements%3A_The_Alkaline_Earth_Metals/1Group_2%3A_Chemical_Reactions_of_Alkali_Earth_Metals/Group_2%3A_General_Properties
- https://byjus.com/jee/alkaline-earth-metals/

