Hydrogen is a peculiar element in nature, resembling halogens and alkali metals. The preparation of hydrogen gas involves several processes. The various methods for the preparation of hydrogen are as follows:
Interesting Science Videos
1. From acid:
The metals that are more electropositive than hydrogen, such as zinc, iron, magnesium, and so on, react with acid to form hydrogen gas.
Mg + H2SO4 → MgSO4 + H2
Metals in the electrochemical series above hydrogen are strongly electropositive and can rapidly displace hydrogen from dilute acids (HCI and H2S04). The electrochemical series of elements is formed by arranging them in decreasing order of standard oxidation.
Table I: Electrochemical series
| Electrode system | Oxidation potential (EO) | H2 Displacement | Electropositive and electronegative character |
| Li / Li+ K / K+ Ca / Ca 2+ Na / Na+ Mg / Mg2+ Al / Al 3+ Zn / Zn 2+ Fe / Fe 2+ Sn / Sn 2+ Pb / Pb 2+ | + 3.05 + 2.92 +2.87 + 2.71 + 2.37 + 1.66 + 0.76 + 0.44 + 0.14 + 0.13 | The tendency of a metal to displace hydrogen from dilute acid increases ↑ | The tendency of a metal to displace hydrogen from dilute acid increases↓ |
| H2 / H+ | 0.00 | ||
| Cu / Cu 2+ Hg / Hg 2+ Ag / Ag + Br–/ Br2 Cl–/ Cl2 F–/ F2 | + 0.34 + 0.79 + 0.80 -1.09 -1.36 -2.87 | No tendency to displace hydrogen from dilute acid |
(Table-I An element having high oxidation potential is reductant and it can displace
cation of high reduction potential into a free state by reduction. Therefore metal atoms above hydrogen can reduce the H+ of acid to H2 whereas the metal atoms below hydrogen in an electrochemical series such as Cu, Hg, Ag, etc can’t do so due to their oxidation potentials lower than that of hydrogen atoms.
Important points
- Pure zinc is not utilized as it reacts slowly with dilute sulphuric acid. While using pure zinc, a few drops of CuS04 solution should be added to accelerate the reaction.
- Commercial zinc contains impurities such as Cu, Cd, and others which help to accelerate the process by producing an electrochemical couple. As a result, commercial zinc is used for the preparation of hydrogen from acid.
- The acids utilized in the production of hydrogen gas should be mild oxidizing acids. Concentrated H2SO4 and HNO3 at any dilution cannot be applied to produce hydrogen because they readily oxidize hydrogen into water as:
4 Zn + 10 HNO3 → 4 Zn (NO3)2 + N2O + 5 H2O
Cu + H2SO4 → CuSO4 + 2 H2O + SO2
Laboratory preparation
Principle
In the laboratory, hydrogen gas is typically produced by the action of dilute acids (HCl and H2SO4) on certain metals (Zn, Fe, and so on). Cavendish used this approach to create hydrogen in the laboratory.
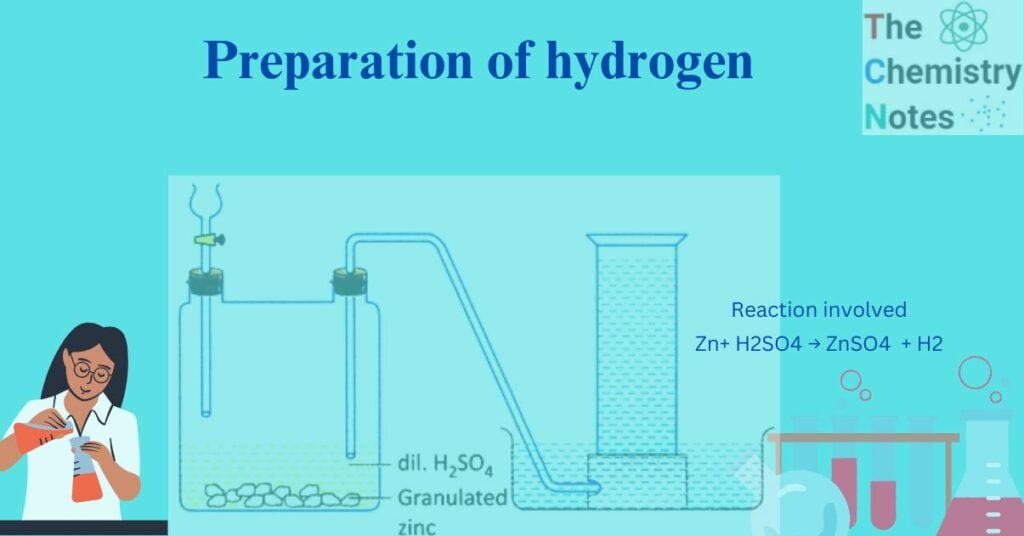
Zn+ H2SO4 → ZnSO4 (aq) + H2
Procedure
Hydrogen gas is produced in Woulfe’s bottle by a reaction between dilute sulphuric acid and granulated zinc at ordinary temperature. Thus produced hydrogen gas is allowed to pass through the delivery tube and collected in a gas jar downward displacement of water because the gas is almost insoluble in water.
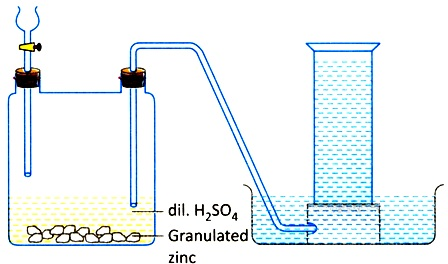
Laboratory preparation of hydrogen
Image source: https://www.embibe.com/question-The-apparatus-is-set-up-for-the-preparation-of-a-certain-gas-in-the-laboratory.-Which-gas-is-this%3F%0A/EM6104751
Zinc is generally used for the laboratory preparation of hydrogen due to following reasons:
- Sodium and potassium react violently with acid.
- Calcium and magnesium are expensive and immediately produce hydrogen.
- Because of its high affinity for oxygen, aluminum is coated with Al203. This layer prevents the metal from reacting with dilute acids.
- When lead is subjected to sulphuric or hydrochloric acid, it forms an insoluble covering of lead sulfate or lead chloride, blocking further reaction.
- Iron interacts slowly with acids to produce hydrogen.
Purification of hydrogen
The hydrogen produced by the action of cold dil. H2S04 on commercial zinc is not pure. It contains certain gaseous contaminants such as arsenic hydride (AsH3), phosphine hydrogen sulfide (H2S), sulfur dioxide (So2), and some nitrogen and carbon oxides such as NO and CO2, as well as some moisture: Various impurities, their sources, and removal methods are tabulated below:
| Impurities | Source | Removal method | Reaction involved |
| H2S | H2SO4 and Zn | By passing gas through lead nitrate solution | H2S + Pb(NO3)2 → PbS + 2 HNO3 |
| PH3 | Zn | AgNO3 or Ag2SO4 solution absorbs it | PH3 + 3 AgNO3 → Ag3 P + 3 HNO3 |
| ASH3 | Zn | CO2, SO2, and oxides of nitrogen | AsH3 + 3 AgNO3 → Ag3As + 3 HNO3 |
| By passing gas through the KOH solution | H2SO4 | Water vapor and moisture | a. SO2 + 2KOH → K2SO3 + H2O b. CO2 + 2KOH → K2CO3 + H2O c. 2NO2 + 2KOH → KNO2 + KNO3 + H2O |
| By passing gas through the P2O5 solution | H2SO4 | By passing gas through P2O5 solution | 3 H2O + P2O5 → 2 H3PO4 |
Test for hydrogen
To determine whether the created gas is hydrogen or not, we must insert a lighted splinter into the gas’s mouth. The gas burns off with a remarkably pale-blue flame near the jar’s mouth, producing a pop sound.
Precaution for hydrogen preparation in the laboratory
I. The thistle funnel’s end should be dipped into the acid layer.
II. Since hydrogen reacts explosively with oxygen in the air, the equipment must be airtight, and the flame should not be placed near it.
2. Preparation from water
a. From cold water
When alkali, alkaline earth metals, and their hydrides react with cold water, hydrogen is produced. Very active metals, such as Na, K, and Ca, react with cold water to liberate hydrogen. The reaction with alkali metals such as Na and K is so intense and exothermic that the hydrogen gas produced catches fire. Generally, amalgams of these metals are utilized to slow down the process.
2 Na + 2 H2O → 2 NaOH + H2
2 K +2 H2O → 2 KOH + H2
Ca + 2 H2O → Ca(OH)2 + H2
NaH + H2O → NaOH + H2
The reaction between sodium or potassium and water is violent, and the reaction rate is reduced or regulated by performing the reaction in the form of amalgam with water.
2Na-Hg + 2 H2O → 2 NaOH + H2
b. From boiling water
Certain metals, such as zinc, magnesium, and aluminum, decompose the boiling water, producing hydrogen gas.
Zn + H2O → Zno + H2
Mg + H2O → MgO + H2
2Al + 3 H2O → Al2O3 + H2
c. From steam
Many metals, including iron, tin, and nickel, may decompose steam at high temperatures, producing hydrogen gas.
3 Fe + 4 H2O ( Red hot steam) ⇋ Fe3O4 ( ferresoferic oxide) + 4 H2
Ni + H2O ( Hot steam) → NiO + H2
3. Preparation from alkalies
Hydrogen can be prepared by boiling caustic soda with metals like zinc, tin, and aluminum, or non-metals like silicon.
Zn + NaOH → Na2ZnO2 ( sodium zincate) + H2
Sn + 2 NaOH → Na2SnO2 ( sodium stannate) + H2
Si + 2 NaOH + H2O → Na2SiO3 ( sodium silicate) + 2 H2
4. Industrial preparation of hydrogen
Raw materials for industrial processing should be inexpensive and readily available. Water, hydrocarbons, and coke are the primary ingredients utilized in the large-scale preparation of hydrogen gas.
The following are some of the most important methods of preparation:
I. By electrolysis of water
Electrolysis is typically performed in a specific vessel known as an electrolytic cell or voltameter.
In areas where electricity is inexpensive, the preparation of hydrogen is carried out by the process known as the electrolysis of water. A tiny amount of dilute acid is added to the voltameter containing water to generate a strong electrolyte. In this electrolytic cell (voltameter), iron works as a cathode, while nickel-plated iron serves as an anode. An asbestos diaphragm separates these two electrodes, preventing hydrogen and oxygen gas from interacting with one another When an electric current is passed through the system, hydrogen forms at the cathode, and oxygen forms at the anode.
Reaction involved:
2 H2O → O2 + 2 H2
H2O ⇌ H + + OH–
At cathode:
H + + e– → H (atomic hydrogen)
H + H → H2 ( molecular hydrogen)
At cathode:
OH– + e– → OH
4 OH → 2 H2 O + O2
II. From red hot iron and steam (Lanes’ process of preparation of hydrogen)
When steam is passed over iron heated to a temperature of 600-8500C, hydrogen is released. This step is known as the oxidation step.
3Fe + 4 H2O ⇌ Fe3O4 + 4 H2 + 38.4 kcal
The process is reversible, and the resulting ferrosoferric oxide can be reduced back to metallic iron by passing water gas (CO + H2) over it. This is also called the reduction step.
Fe3O4 + 2 (CO + H2 ) → 3 Fe + 2 H2O + 2 CO2
As a result, the same amount of iron is used repeatedly to produce hydrogen gas from steam. it is a commercial method of preparation of hydrogen.
III. From water gas
Water gas, a mixture of carbon dioxide and hydrogen gas, is also an inexpensive source of commercial hydrogen. In the first phase, water gas is created by flowing steam over red hot coke. Hydrocarbon can also be used in place of coke.
C + H2O → ( H2 + CO) – 29 kcal
CH4 + H2O → CO + 3 H2
Hydrogen gas be prepared from water gas by using the following two methods:
a. By liquefaction
Carbon monoxide liquefies at -190 degrees Celsius, while hydrogen liquefies at -253 degrees Celsius. Under suitable conditions, carbon monoxide liquefies, but hydrogen remains as gas when a mixture is subjected to high pressure and low temperature. Uncondensed hydrogen is collected in cylinders.
b. By converting CO into CO2 ( Bosch process of preparation of hydrogen)
Water gas is mixed with more steam, and the mixture is then passed over a heated catalyst (a mixture of iron oxides) at 450-500 0C with appropriate promoters such as Al, Cr, Ni, Ce, or Th oxides. In this process, carbon monoxide is converted to carbon dioxide.
( H2 + CO) + H2O ( steam) ⇌ 2 H2O + CO2 + Heat
The resultant mixture contains traces of CO, as well as CO2 and hydrogen. The mixture is compressed under high pressure (25 atms) and then run through cold water to dissolve the carbon dioxide. When 99.9% hydrogen is achieved, the oxidation and absorption process is performed twice.
CO2 + H2O → H2CO3 ( carbonic acid)
The remaining CO is eliminated by passing the gas through ammoniacal cuprous formate.
IV. from the methane steam process
Hydrogen gas is manufactured when a mixture of methane and steam is passed over a heated nickel catalyst at 1200⁰C and compressed up to 30 atmospheres. Methane is obtained as a byproduct of the petroleum industry.
The chemical reaction involved in this process is given below:
CH4 + H2O → CO + 3 H2
V. Electrolysis of barium hydroxide solution
The warm aqueous solution of barium hydroxide is electrolyzed between the platinum or nickel electrodes to obtain the high purity of hydrogen. The gases produced in the anode and cathode compartments must be separated. The following reactions occur at the cathode and anode:
At anode:
2OH– → H2O + 1/2 O2 + 2e–
At cathode:
2 H2O + 2e– → 2 OH– + H2
The overall reaction is:
H2O → H2 + 1/2 O2
V. As a by-product
Hydrogen is produced as a by-product during the manufacture of NaOH from brine ( aqueous NaCl) by the process of electrolysis.
NaCl ⇌ Na+ + Cl–
H2O + 2 e → H2 + 2 OH–
Na+ + OH– → NaOH
2 Cl– – 2 e → Cl2
Chemical properties of hydrogen
I. Action with litmus
It is neutral to litmus.
II. Combustibility
Although hydrogen is a combustible (inflammable) gas, it does not promote burning.
When it burns in air or oxygen, it creates water. As a result, the word hydrogen signifies “water producer.”
2 H2 + O2 → 2 H2O
When a lighted splinter is placed in a jar containing hydrogen, it is extinguished in the lack of oxygen in the air, but in the presence of oxygen, a gas of hydrogen burns, generating a pop sound. It is a hydrogen test.
III. Action with non-metals
Under suitable conditions, hydrogen combines with most of the non-metals to form stable compounds.
2 H2 + O2 → 2 H2O ( at 800 OC)
H2 + F2 → 2 HF ( Dark – 250 OC)
H2 + I2 → 2 HI ( 440 OC)
H2 + Br2 → 2 HBr ( 440 OC)
IV. Action with metals
Hydrogen reacts with strongly electropositive metals like lithium, sodium, potassium, calcium, etc to give metal hydride. In these reactions, hydrogen acts as an oxidizing agent.
H2 + 2 Na→ 2 NaH (at 300 OC)
H2 + Ca → CaH2(at 300 OC)
V. Action with metal oxides
When hydrogen passes over heated metallic oxides, it removes the oxygen from them to form water, reducing the metallic oxides to their metallic state.
Fe3O4 + 4 H2 → 3 Fe + 4 H2O
CuO + H2 → Cu + H2O
PbO + H2 → Pb + H2O
VI. Action with carbon monoxide
Methanol is produced by passing a mixture of carbon monoxide and hydrogen through heated ZnO and Cr2O3 at around 300 oC and 200 atm.
CO + 2 H2 → CH3OH
VII. Catalytic hydrogenation
Catalytic hydrogenation is the addition of hydrogen to multiple bonds in the presence of a catalyst. The reduction of a chemical using hydrogen, for example, the preparation of ethane from acetylene, is known as hydrogenation.
When hydrogen gas is fed over liquid oils with finely divided nickel as a catalyst, the oils absorb the hydrogen and convert it to fats. This is known as the hydrogenation of oil and is utilized in the production of Vanaspati ghee (vegetable ghee).
Vegetable oil + H2 → Solid fat ( at 8-10 atm/ 200 0C , Ni)
This process is also known as the hardening of oil. The hydrogenation process is commonly observed in the case of organic reactions. For example, ethylene, an unsaturated hydrocarbon of the formula, C2H4 is converted into the corresponding saturated hydrocarbon ethane C2H6 when a mixture of ethylene and hydrogen passed over finely divided nickel at 200 0C.
C2H4 + H2 → C2H6
References
- J. D. Lee (1977). A new concise inorganic chemistry (3d ed.). Van Nostrand Reinhold. Retrieved May 24 2023 from https://archive.org/details/newconciseinorga00leej.
- S.D. Gautam, M. Pant and N.R. Adhikari (2072). Comprehensive Chemistry Part 1. 6th edition. , Heritage Publishers and Distributors, Bhotahity, Kathmandu.
- https://byjus.com/chemistry/laboratory-preparation-of-hydrogen-gas/.
- https://www.britannica.com/science/hydrogen/Production-and-applications-of-hydrogen.
- https://www.allaboutchemistry.net/preparation-hydrogen-laboratory-industrial/
