Metallurgy is a scientific process that employs a range of techniques to extract metals in their pure form from ores. Metallurgy encompasses a set of principles and technological procedures utilized for the extraction and purification of metals, ultimately leading to the production of highly pure metal.
Interesting Science Videos
General Principles of Extraction of Metals [Principles of Metallurgy]
With the exception of native ores in specific locations, the majority of metal ores are typically found in a combined state with other electronegative elements, forming various compounds. To achieve metal extraction in its pure form, it is necessary to thoroughly eliminate all impurities, the matrix, and any elements that remain in a combined state with the metal within the ore. The selection of extraction principles and technological operations is contingent upon the characteristics of the ores and impurities, as well as the properties of the metal.
Typically, three primary metallurgical procedures exist employed to extract a metal from its ore. The following operations are included:
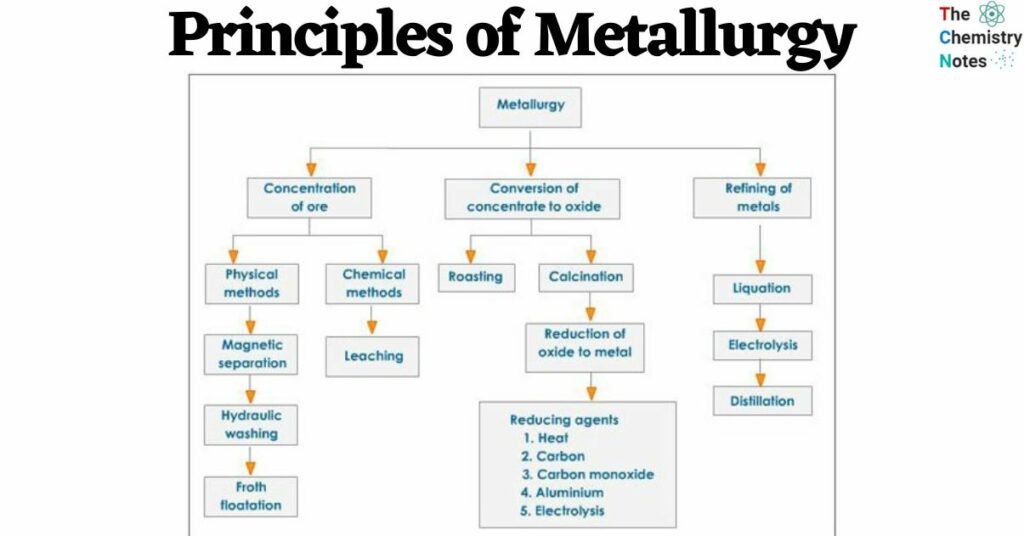
(1) Ore Dressing And Concentration
(2) Extraction of Metal From Concentrated Ore
(3) Purification of Metal
General principles involved in each operation are outlined below based on the type of ore, metal, and contaminants being processed.
Ore Dressing And Concentration
The initial step involves breaking down and grinding the provided ore, aiming to achieve a finely powdered form through crushing and pulverizing technology. Afterward, the particular techniques of concentration are implemented, considering the characteristics of the ore and any accompanying impurities.
Washing And Gravity Separation
The process involves the deposition of powdered ore on inclined decks or tables, followed by the application of water jets. The water flow effectively carries away the lighter constituents, such as clays and gangue mud, through this action. Consequently, the heavier ore particles remain behind, facilitating their isolation. Gravity separation is a commonly employed technique for the concentration of oxide ores, such as tinstone (SnO2), haematite (Fe2O3), and bauxite (Al2O3.2H2O), among others. Gravity separation is a viable method for the concentration of heavy metal ores, such as those containing gold (Au), silver (Ag), and others.
Froth-Floatation Process
In the process, finely crushed ore, specifically sulfide ore, is thoroughly combined with water, along with a minute quantity of xanthate oil, which serves as a foaming agent, and eucalyptus or pine oil, which acts as a frothing agent, within a container.
The mixture undergoes churning by means of air that flows through the nozzles, as depicted in the diagram.
Sulfide ore particles exhibit a propensity to attract air and oil, resulting in the formation of froth that remains buoyant on the surface. Conversely, non-sulfide earthen impurities become saturated with water and precipitate at the bottom of the tank. In this particular case, the formation of froth facilitates the concentration of the sulfide ore, thereby simplifying its separation from the gangue.
The foam obtained during the collection process is subjected to acid treatment in order to disperse the foam. The froth-flotation process is utilized for the concentration of sulfides and pyrites, including elements such as copper (Cu), zinc (Zn), lead (Pb), silver (Ag), and others.
Electromagnetic Separation
Electromagnetic separation can be employed to enhance the concentration of an ore when the impurities present in the ore exhibit comparable densities and magnetic properties.
Hence, the mineral known as tinstone, which is primarily composed of tin, contains wolfram (FeWO4) as an incidental constituent. It is noteworthy to mention that Wolfram possesses paramagnetic properties and demonstrates an inclination towards magnetic attraction.
The application of powdered tinstone onto a conveyor belt that traverses electromagnetic rollers will lead to its subsequent removal.
The paramagnetic properties of tungsten result in its magnetic attraction, leading to its accumulation in close proximity to magnets. In contrast, tinstone demonstrates divergent behavior by segregating from the wolfram, resulting in the formation of a discernible accumulation.
Leaching Process
The aforementioned procedure is a form of chemistry that constitutes one aspect of hydrometallurgy, especially applied for the objective of ore concentration. The powdered ore undergoes treatment with an appropriate reagent. The ore undergoes dissolution, whereas the impurities remain in an undissolved state. The aforementioned procedure is commonly referred to as leaching.
An illustration of this process involves the utilization of a concentrated NaOH solution to treat bauxite, resulting in the dissolution of aluminum oxide while insoluble impurities remain. These impurities are subsequently eliminated through the process of filtration.
Al2O3.2H2O + 2 NaOH → 2 NaAlO2 (sod. meta aluminate (water soluble)+ 3 H2O
NaAlO2 + 2 H2O → Al(OH)3↓ + NaOH
2 Al(OH) → Al2O3+3 H2O
Metal Extraction From Concentrated Ore
The subsequent process entails the preparation of the concentrated ore for metal extraction, which can be achieved using any of the following four techniques:
(I) Preliminary Heating (Calcination And Roasting)
(II) Alumino Thermic Process of Reduction
(III) Electro-metallurgic Reduction
(IV) Hydrometallurgy Reduction
Here we will be discussing the above-mentioned methods in detail.
Preliminary Heating
The process comprises the subsequent operations. The process entails the conversion of ore into oxide ore, which is subsequently reduced to obtain the metal by employing a suitable reductant. The process consists of the following steps:
(a) Preliminary Heating (Calcination And Roasting)
(i) Calcination
Roasting is an initial process wherein concentrated ore is subjected to heating in an environment devoid of or with a restricted air supply, with the aim of attaining a temperature lower than its melting point. The objective of this procedure is to remove volatile impurities while minimizing the occurrence of oxidation. The usual practice involves the utilization of a reverberatory furnace. Calcination has the potential to induce a multitude of alterations. The changes that are occurred by calcination are as follows:
The process involves the elimination of moisture and other volatile impurities.
The organic matter undergoes degradation and subsequent elimination.
The process of dehydration involves the extraction of water from hydrated and hydroxide ores. Carbon monoxide (CO) and carbon dioxide (CO2) are extracted from carbonate ores.
Al2O3.2H2O → Al2O3+2 H2O↑
ZnCO3 → ZnO + CO2↑
CuCO3.Cu(OH)2 → 2 CuO+ CO2↑ + H2O↑
The calcined ore undergoes a transformation, resulting in a soft and porous form, allowing the processing of it in subsequent stages.
(ii) Roasting
Roasting refers to the thermal treatment of concentrated ore in the presence of an excess of oxygen from the air, wherein the temperature is deliberately maintained below the melting point. This controlled heating process aims to eliminate volatile impurities through oxidation. The process is conducted within a reverberatory furnace. Several alterations take place during the roasting process.
The process involves the removal of organic matter through oxidation and the elimination of moisture.
The process involves the formation of oxides to eliminate volatile substances such as carbon (C), sulfur (S), phosphorus (P), and others.
C + O2 → CO2
S + O2 → SO2
4 P + 5 O2 → 2 P2O5
The process eliminates sulfur dioxide (SO2) from sulfides, pyrites, and sulfate ores.
2 ZnS + 3 O2 → 2 ZnO + 2 SO2
4 FeS2 + 11O2 → 2 FeO3 + 8 SO2
2 ZnSO4 → 2 ZnO + 2 SO2 + O2
Additionally, the roasting process induces porosity in the mass, thereby facilitating its handling in subsequent stages.
Smelting
Smelting refers to the procedure of subjecting roasted, calcined ore to high temperatures in the presence of a reducing agent, typically charcoal, and an appropriate flux. This process is carried out above the melting point of the ore. The process of smelting typically encompasses:
- The process of reducing metal using carbon
- Impurity Removal through Slag Formation
The heat required for the process is generated within the furnace through the combustion of carbon (C) using a high-temperature stream of air. The furnaces employed in this process are either blast furnaces or reverberatory furnaces.
(i) Reduction With Carbon
Metal oxides, obtained through the processes of calcination or roasting, are mixed with carbon to undergo reduction and convert into metal using a dry extraction technique. The procedure mentioned above is frequently known as the carbon reduction process. The following examples are provided below.
ZnO + C Δ → Zn + CO
CuO + C Δ → Cu + CO
SnO2 + 2 C Δ → Sn + 2 CO
Fe2O3 + 3 C Δ → 2 Fe + 3 CO
Carbon monoxide (CO) can be generated through incomplete combustion of carbon, leading to the reduction of the oxide to its elemental form.
Fe2O3 + 3 CO Δ → 2 Fe + 3 CO2
(i) It is the most cost-effective among all the provided reducing agents.
(ii) The substance can be readily pulverized and effectively blended.
(iii) Carbon monoxide (CO) can be created as a gaseous reducing agent as well as subsequently utilized for the reduction of the metal oxides found in the upper section of the furnace.
(iv) The process of carbon combustion is known for its significant release of heat energy, making it a suitable source for providing the necessary thermal energy in the smelting process.
C + O2 → CO2 + 94.05 kcal
(v) The waste gas, which is the final product (CO2) resulting from metallurgical reduction, is self-removed.
The carbon reduction process is not suitable for extracting alkali metals, alkaline earth metals, and aluminum due to their propensity to form stable carbides through their affinity for carbon.
(ii) Impurity Removal through Slag Formation
In numerous instances, alongside carbon, supplementary substances known as flux are introduced to facilitate the elimination of non-melting impurities (referred to as matrix) by forming a molten mass called slag.
Typically, due to its immiscible nature and lower density compared to molten metal, the slag flows to the surface and can be readily removed through blowing or runoff.
As an example, the removal of silica can be achieved through the addition of lime as a flux.
CaO + SiO2 → CaSiO3
Flux Impurity Slag
Flux is a substance that is introduced to ores prior to their heating in order to facilitate a chemical reaction with the impurities, also known as gangue, found within the ores. This reaction results in the formation of slag that is capable of melting at a lower temperature. There are three distinct types of fluxes:
(i) Acidic fluxes such as silica (SiO2), borax (Na2B4O7), P2O5, and others, are commonly used in various applications.
(ii) Basic fluxes include limestone (CaCO3), magnesite (MgCO3), haematite (Fe2O3), and others.
(iii) Neutral fluxes such as calcium fluoride (CaF2), are commonly employed to preserve the fluidity of molten substances.
Gold Schmidt’s Alumino Thermic Process
- Due to the higher affinity of oxygen for certain oxides compared to carbon, carbon is unable to reduce them.
- Metals can be reduced using aluminum powder.
- The oxide is mixed with aluminum powder and a small amount of barium peroxide (BaO2) before being placed in a sizable container.
- Barium peroxide functions as an oxidizing agent, thereby aiding in the initiation of combustion.
The charge is initiated by igniting a magnesium ribbon placed in a pocket containing a mixture of barium peroxide and powdered magnesium, as shown in the figure. A reduction reaction occurs, converting oxide into its corresponding metal form. For example,
Cr2O3 + 2 Al → Al2O3 + 2 Cr + 112 kcal
3 MnO2 + 4 Al → 2 Al2O3 + 3 Mn + heat
3 Mn3O4 + 8 Al → 4 Al2O3 + 9 Mn + heat
The significant heat generated causes the metal to melt, while Al2O3 remains unaffected. Consequently, the molten metal accumulates beneath the alumina layer in the crucible.
Electromtallurgic Reduction
The use of aqueous solutions containing salts derived from alkali, alkaline earth metals, and aluminum obtained from their ores is not feasible for electrolytic reduction. This phenomenon occurs because the active metals produced during the process undergo a reaction with water, leading to the creation of their respective hydroxides and hydrogen.
2 Na + 2 H2O → 2 NaOH + H2↑
i.e., the H+ of the aqueous solution is reduced in preference to those metal ions.
The feasibility of utilizing carbon for the reduction of active metal oxides is hindered by the necessity of maintaining high temperatures. When exposed to high temperatures, the element carbon undergoes a chemical reaction with various metals, leading to the creation of carbides that exhibit long-term stability.
Ca + 2 C → CaC2
4 Al + 3 C → Al4C3
- Hence, metals with high reactivity are typically obtained through the process of electrolysis, whereby their appropriate salts are subjected to fusion. The cathode facilitates the deposition of pure metal.
- Aluminum is acquired through the process of electrolysis, specifically by subjecting fused alumina to this method.
- Similar to how sodium and calcium are both obtained through the electrolysis of fused sodium chloride and caustic soda, so is calcium.
NaCl ⇌ Na+ + Cl–
At cathode: Na+ + e– → Na
At anode: Cl– – e– → Cl
Cl + Cl → Cl2
Supplementary substances, which do not undergo prior reduction, are introduced to decrease the melting point or enhance conductivity. Sodium (Na), Aluminum (Al), Magnesium (Mg), and Calcium (Ca), as well as elements belonging to Group IIIB and the lanthanides, are typically obtained through the process of electrolysis. This method involves the utilization of their molten salts, commonly in the form of chlorides.
Hydrometallurgy Reduction
Noble metals such as silver (Ag) and gold (Au) are commonly extracted from their ores using hydrometallurgical methods. One effective technique for precipitating these metals involves the introduction of highly electropositive metals. The electropositive metals should be positioned at a higher level compared to the noble metals in the electrochemical series. Silver is acquired by introducing zinc into a sodium argento cyanide solution.
2 Na[Ag(CN)2] + Zn (Scrap Zinc) → 2 NaCN + Zn(CN)2 + 2 Ag
or, Ag2S + 2 NaCN → 2 AgCN + Na2S
AgCN + NaCN → Na[Ag(CN)2]
[Na[Ag(CN)2] ⇌ NaCN + AgCN x 2
2 AgCN + Zn → Zn(CN)2 + 2 Ag
2 Na[Ag(CN)2] + Zn → 2 NaCN + Zn(CN)2 + 2 Ag↓
or, 2 Na[Ag(CN)2] + Zn → 2 Ag + Na2[Zn(CN)4] sodium tetracyano zincate
How to Select Flux
The selection of the flux is contingent upon the type of impurities that are present. In the case where impurities possess acidic properties, such as sand or P2O5, it is necessary to employ basic fluxes like lime or magnesia.
SiO2 + CaCO3 → CaSiO3 + CO2
P2O5 + CaCO3 → Ca3(PO4)2 + 3 CO2
When the gangue exhibits basic properties, it is necessary to use an acidic flux such as silica (sand).
CaO + SiO2 Δ → CaSiO3
MnO + SiO2 Δ → MnSiO3
FeO + SiO2 Δ → FeSiO3
Gangue Flux Slag
What is Slag?
The fusion of flux and gangue produces slags, which are useful substances. In metallurgical processes, they are easy to get rid of because fused slag and molten metal don’t mix. This makes different layers at the bottom of the furnace that are easy to separate. Slags are predominantly composed of silicates resulting from the reaction between sand and various bases such as FeO, CaO, Al2O3, MnO, and others.
References
- https://byjus.com/chemistry/processes-of-metallurgy/#
- https://study.com/learn/lesson/metallurgical-process-principles-examples.html
- https://pratibha.eenadu.net/admissions/lesson/neet/general/english-medium/education/1-2-15-0-839-577-4970-6063-1078-20040006761
- https://chem.libretexts.org/Bookshelves/General_Chemistry/Map%3A_General_Chemistry_(Petrucci_et_al.)/23%3A_The_Transition_Elements/23.2%3A_Principles_of_Extractive_Metallurgy
- https://www.chemicalslearning.com/2021/12/Types-of-Metallurgy-Process.html
