When colloidal solutions are prepared, they typically contain an excessive number of electrolytes, as well as some other soluble impurities. Despite the presence of traces of electrolytes required for colloidal solution stability, larger amounts coagulate it. As a result, it is necessary to reduce them. The process of reducing the amount of impurities to the minimum level is known as the purification of colloids.
Interesting Science Videos
Different methods of purification of colloids
There are different methods for the purification of collides they are:
Dialysis
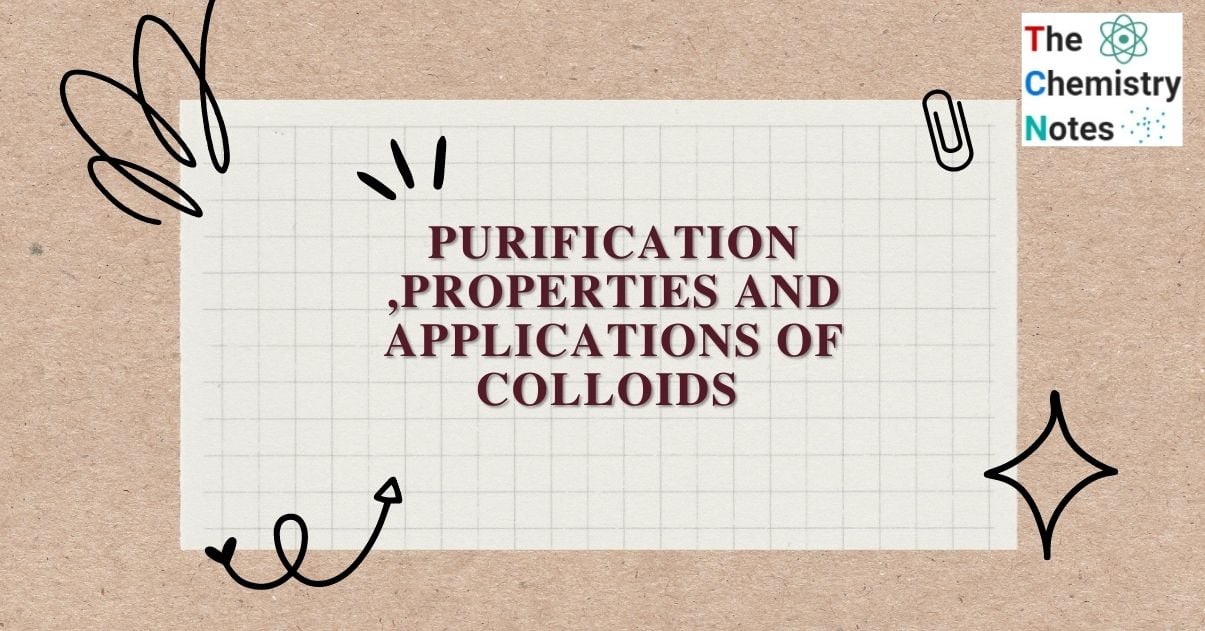
It is the removal of a dissolved substance from a colloidal solution via diffusion through a suitable membrane. The colloidal solution is placed in a cellophane bag that is suspended in a tub of fresh water. Impurities diffuse out of the bag, leaving only pure colloidal solution. The dialysis process is based on the fact that colloidal particles cannot pass through a parchment or cellophane membrane, whereas the ions of the true solution can pass through it.
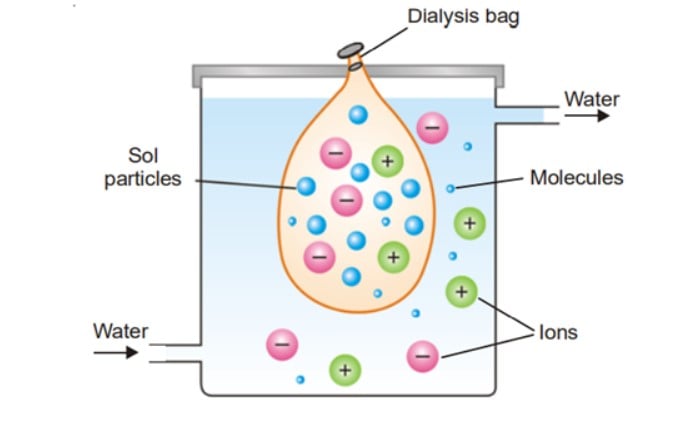
Electrodialysis
Dialysis is a slow process that is accelerated by the presence of an electrical field. When an electric field is applied to the electrodes, the ions of the electrolyte present as impurity diffuse rapidly towards oppositely charged electrodes. The artificial kidney machine work on the principle of dialysis.
Ultrafiltration
Ultrafiltration is the separation of colloidal particles in a colloidal solution using specially prepared filters. The ultra-filter paper only filters the dispersed phase, while the dispersion medium and other soluble impurities pass through. As the colloidal particles can pass through the normal filter paper, the ultra-filter paper with a pore size of 0.0015 micrometers is used for this purpose.
Ultra-centrifugation
This process involves the separation of the colloidal particle using a centrifugation tube. The colloidal solution s placed in the centrifugation tube.
When the tube is rotated quickly, the colloidal particles settle at the bottom, while the impurities remain in the solution. The pure sol is regenerated by adding a suitable dispersion medium to the particles settled at the bottom.
Precipitation of colloid
The presence of charge on colloidal particles accounts for the lyophobic sols’ stability. If the charge is removed in some way, the particles will come closer together to form aggregates (or coagulate) and settle down. The process of settling colloidal particles is known as coagulation or sol precipitation. The colloidal particles can be coagulated by different methods they are:
- By electrophoresis: Electrophoresis is the process in which the colloidal particles are discharged and precipitated as they move towards oppositely charged electrodes.
- By mixing two oppositely charged sols: When oppositely charged sols are mixed, their charges neutralize and they precipitate.
- By boiling: When a sol is boiled, the adsorbed layer is disturbed due to increased collisions with dispersion medium molecules. This reduces the charge on the particles, causing them to settle and form a precipitate.
- By persistent dialysis: During prolonged dialysis, the colloid becomes unstable and eventually coagulates.
- Addition of electrolytes: When the electrolyte is added to the sol, the charge present in sol particles gets discharged due to ions furnished by the electrolytes. A positive ion causes precipitation of negatively charged sol and vice versa.
Hardy Schultze rule
Oppositely charged ions from the electrolyte are used up for coagulation or precipitation of sol particles. The precipitating power of ions depends on their valency. According to the Hardy-Schultze rule, the higher the valency of active ions, the greater will their power to coagulate colloidal solution.
In the coagulation of a negative sol, the coagulating power of ions is in the order:
Al3+ >Ba2+ >Na+
Similarly, in the coagulation of a positive sol, the coagulating power of ions is in the order:
[Fe(CN)6] 4– > PO4 3– > SO4 2– > Cl –
Flocculation and Flocculation value
Coagulation is the process in which the charge over the colloidal particles is neutralized resulting in the precipitation of colloidal particles. This process is brought about by the addition of electrolytes. Thus, the colloidal particles accumulate to form particles of larger size and coagulation results. If the coagulated substance floats, then is called flocculation.
The number of millimoles of an electrolyte that is to be added to one liter of the colloidal solution to cause complete precipitation is called as flocculation value. The smaller the flocculation value the higher the precipitating power of an ion.
Coagulation value or flocculation value ∝ 1/ Coagulating power
Origin of charge on sol particles
All the dispersed particles of a particular sol carry a positive or a negative charge depending on the nature of sol particles. They acquire this charge by
1. Adsorption of ions from the aqueous solution
2. Ionization of surface group
Adsorption of ions from the aqueous solution:
In most cases, the charge on the sol particles originates from the adsorption of particles common to the particles from the dispersion medium. For example:
Ferric hydroxide sol particles are positively charged because they adsorb the common ions Fe 3+ from the aqueous solution.

The particles may absorb the cations or anions which are present in excess in the solution. For example, AgCl sol obtained from the addition of AgNO3 solution to the sodium chloride bears a positive charge if Ag+ ions are in excess. On other hand, if there is the presence of excess Cl- ions then it bears a negative charge.
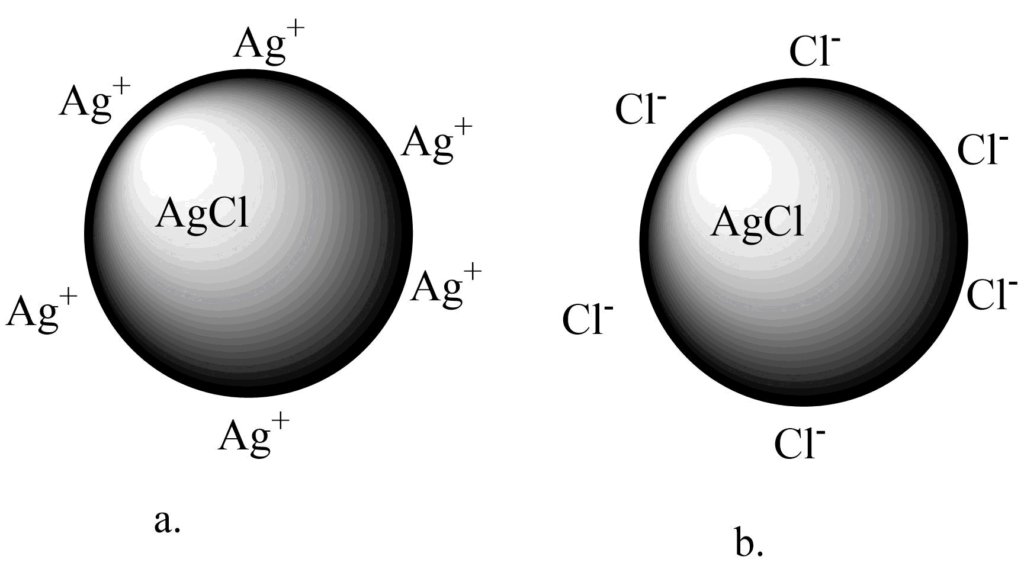
Fig 4: Origin of charge on AgCl sol particle
Ionization of surface group
Charge on the surface-active agents or surfactants originates due to the ionization of surface groups. For example:
- Origin of charge on soap and detergent sols
- Origin of charge on protein sols.
Stability of colloids
Colloidal solutions are electrically charged so they are stable. Colloidal particles either carry a positive or negative charge. Since all colloidal particles bear identical charges, they repel each other and do not precipitate easily under the effect of gravity.
Both lyophilic and lyophobic sol particles are identically charged so the electrostatic repulsion will be created. As a result, the particles can not come closer, and hence they do not combine. But in the case of a lyophilic sol, there is a better solvent particle attracting affinity so that lyophilic sol particles are sheathed with solvent particles which makes them more stable as compared to lyophobic sol.
Protection of colloids and gold number
Lyophilic sols have greater stability than lyophobic sols. The addition of a small amount of lyophilic sol increases the stability of lyophobic sol. When lyophilic sol is added to lyophobic sol, it surrounds the lyophobic sol particles and changes the lyophobic nature of the sol into lyophilic. This, in turn, increases the stability of lyophobic sol. So the phenomenon of preventing coagulation of a lyophilic sol by the addition of a lyophilic colloid is known as sol protection or protection of colloid. Those colloids which prevent the coagulation of other less stable colloids are called protective colloids. Gelatin, albumin, and starch are common examples of protective colloids. The protective power of various protective colloids is different. The protective action of the colloids is measured in terms of gold number.
Gold number
The term gold number is first introduced by Zsigmondy to describe the protective power of different colloids. The gold number is defined as the number of milligrams of protective colloid which just prevent the coagulation of 10 ml of gold sol when 1 ml of 10% sodium chloride is added to it. The lesser the value of gold number higher will be the protective power of colloids.
Properties of colloids
Colligative property
Due to the formation of associated molecules, the number of particles in a colloidal solution is comparatively small as compared to a true solution. The observed values of colligative properties such as a relative decrease in vapor pressure, elevation in boiling point, depression in freezing point, and osmotic pressure are smaller than the true solution.
Physical Properties
Heterogenous nature
Colloidal sol consists of a dispersed phase and dispersed medium. They are heterogeneous in nature.
Colour
The color of the colloidal solution is determined by the wavelength of light scattered by the dispersed particles. The wavelength of light is also affected by the size and nature of the particles. The color of the colloidal solution also varies depending on how the light is received by the observer. For example, the finest gold sol is red in color; as particle size increases, it appears purple, blue, and finally golden.
Stability
Colloidal solutions are electrically charged so they are stable. Colloidal particles either carry a positive or negative charge. Since all colloidal particles bear identical charges, they repel each other and do not precipitate easily under the effect of gravity.
Mechanical properties
Brownian movement
In 1827 Robert Brown, a botanist, observed the random movement of pollen grains suspended in water. Later on, the colloidal particles were observed to be in a state of continuous zig-zag pattern. This type of motion is called the Brownian movement. This movement explains the gravitational force acting on the colloidal particles. This movement also contributes to the stability of colloidal sol by preventing it from settling down.
Diffusion
The sol particles diffuse from a higher concentration to a lower concentration region. However, due to their larger size, they diffuse at a lower rate.
Optical properties: Tyndall effect
When the beam of light is passed through the colloidal sol the path of the beam gets illuminated due to the scattering of light by particles. This phenomenon is known as the Tyndall effect. Tyndall effect was first observed by Tyndall in 1869. The bright cone of the light is called the Tyndall cone. The intensity of scattered light is determined by the difference in refractive indices between the dispersed phase and the dispersion medium. Lyophobic sol shows well defined Tyndall effect while in the case of lyophilic sol Tyndall effect is very weak. The Tyndall effect confirms the heterogeneous nature of the colloidal solution. It also helps to distinguish between a colloidal and true solution. The Tyndall effect can be observed under the following conditions:
(i) The diameter of the dispersed particles is not much smaller than the wavelength of the light used.
(ii) The refractive indices of the dispersed phase and the dispersion medium differ greatly in magnitude.
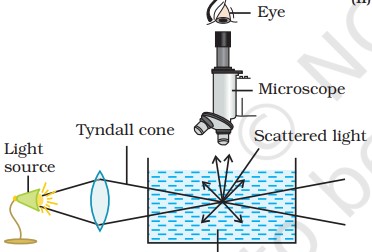
Examples of Tyndall effects are:
- Due to scattering the sky looks blue.
- The blue color of seawater is due to the scattering of blue light by water molecules.
- Visibility of sharp rays of sunlight passing through a slit in a dark room.
suggested video
Electrical properties
Electrophoresis
The movement of the colloidal particles under the influence of electric fields is known as electrophoresis. Electrophoresis is the process in which the colloidal particles are discharged and precipitated as they move towards oppositely charged electrodes.
When the electrophoresis of the colloidal solution is carried out without stirring, the bottom layer becomes more concentrated. The top layer contains pure and concentrated colloidal solution and can be decanted. This is called electro decantation and is used for purification as well as for concentrating the sol.
Helmholtz and Diffuse layer in colloids
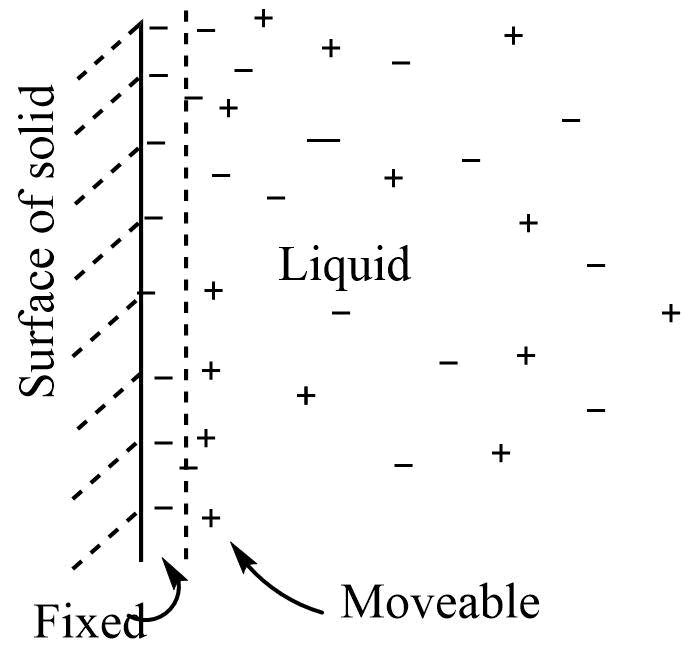
Helmholtz double layer
Any solid in contact with liquid tends to develop a difference in potential across the interface between the two. So when water comes into contact with a glass surface the hydroxyl ions get adsorbed to the glass surface. To counterbalance this charge hydrogen ions are attracted to the surface, resulting in the formation of two layers across the interface. This is called Helmholtz double layer. The layer of charge that is fixed at the interface is called a fixed part of the double layer while the layer that remains on the solution side is called a moveable part of the double layer. The charge on the fixed part is equal and opposite to that on the moveable part so the electrified interface as a whole is neutral.

Diffusion double layer
According to this model, when the solid comes in contact with a liquid dispersion medium, a layer of charge develops on its surface This layer has either a positive or negative charge, which attracts counterions from the solution and forms a diffused layer. The diffuse layer consists of the charge of both signs. The electrified surface as a whole is neutral.
The same type of model can be applied in the dispersed phase and dispersion medium of a colloid to explain the electrical properties of colloids. The ion preferentially adsorbed is held in a fixed part and imparts a charge to colloidal particles.
Zeta potential
The existence of opposite signs on fixed and diffuse parts of the double layer leads to the appearance of a difference in potential. This difference in potential between the two layers is known as zeta potential or electrokinetic potential.
Applications of colloidal solution
Sewage disposal
Sewage on passing through the metallic plate kept on high potential, colloidal particles move to the oppositely charged electrode and precipitate there. As a result, the sewage water is purified.
Medicine
The majority of medicines are colloidal in nature. For instance, Argyrol is a silver sol that is used as an eye lotion.
The blue color of the sky
The colloidal dust particles floating about in the sky scatter blue light, which makes the sky appear blue.
Artificial rain
It is possible to create artificial rain by releasing electrified sand or silver iodide from an airplane, coagulating the mist in the air.
Photographic plate or film
An emulsion of light-sensitive silver bromide in gelatin is coated over glass plates or celluloid films to create photographic plates or films.
Clotting of blood
Blood is a negatively charged colloidal solution. On applying FeCl3 to the wound bleeding stops due to clotting of blood.
Tanning
When a positively charged animal hide is soaked in tannin, mutal coagulation occurs, resulting in leather hardening.
References
- Shaw D. J. (1992). Introduction to colloid and surface chemistry (4th ed.). Butterworth-Heinemann.
- https://byjus.com/chemistry/preparation-of-colloidal-solutions/
- https://ncert.nic.in/ncerts/l/lech105.pdf
- https://www.geeksforgeeks.org/preparation-and-purification-of-colloids/
- https://chemistry.stackexchange.com/questions/128684/why-does-tyndall-scaterring-produce-conical-shape
- https://www.vedantu.com/chemistry/application-of-colloids
- https://www.embibe.com/exams/purification-of-colloidal-solutions/
- https://www.lkouniv.ac.in/site/writereaddata/siteContent/202004070948263098nksingh_Colloidal_State.pdf
- https://nios.ac.in/media/documents/SrSec313NEW/313_Chemistry_Eng/313_Chemistry_Eng_Lesson8.pdf
- https://www.toppr.com/guides/chemistry/surface-chemistry/preparation-of-colloids/#Dispersion_Techniques
- https://byjus.com/chemistry/applications-colloid/#:~:text=A%20colloid%20is%20used%20as,(liquid%2Dsolid%20colloid)
