Organic compounds are complex molecular structures that can consist of multiple elements, and their identification can be achieved through a range of qualitative and quantitative analytical methods. The initial stage in the identification of these compounds entails the discernment of constituent elements, a process commonly referred to as qualitative analysis. It provides insight into the constituent elements that are found within the specific compound in question. The fundamental constituents found in organic compounds encompass carbon, hydrogen, and oxygen. Carbon is consistently and ubiquitously found, hydrogen is predominantly present, and oxygen is typically encountered in organic compounds.
Furthermore, organic compounds may also incorporate nitrogen, sulfur, and halogens, albeit less frequently, while the presence of phosphorous and metals is infrequent in such compounds. Elements that are less frequently encountered in organic compounds, such as nitrogen (N), sulfur (S), and halogens (X), are commonly referred to as foreign elements. Certain organic compounds have the potential to incorporate metallic elements such as sodium (Na), potassium (K), calcium (Ca), magnesium (Mg), iron (Fe), and copper (Cu), among others. This chapter elucidates the identification of exogenous constituents, specifically nitrogen (N), sulfur (S), and halogens (X), namely chlorine (Cl), bromine (Br), and iodine (I), predominantly. Organic compounds are commonly acknowledged to contain carbon and hydrogen, although their detection is also referenced for convenience.
Interesting Science Videos
Detection of Carbon And Hydrogen
- The overwhelming majority of organic molecules primarily comprise carbon atoms, with only a few exceptions. Furthermore, these molecules include hydrogen.
- One instance of a chemical compound that exhibits hydrogen deficiency is carbon tetrachloride. Hence, if the chemical under consideration is identified as being of organic nature, there is no need to conduct tests specifically targeting the carbon and hydrogen elements.
- In order to ascertain the nature of a compound, whether it is classified as organic or inorganic, the analysis involves the detection of carbon and hydrogen.
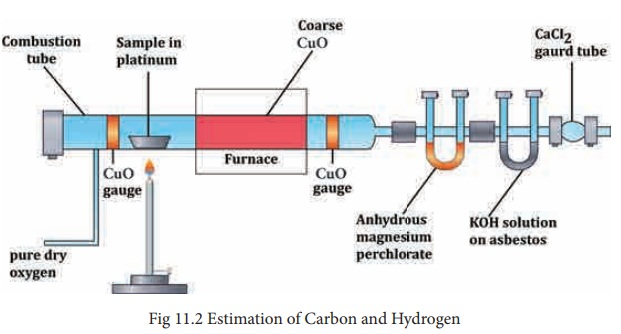
- The presence of carbon and hydrogen can be discerned through the utilization of the copper oxide test. During this experimental procedure, the organic compound undergoes intense heating in the presence of dry cupric oxide within a robust glass test tube.
- The tube is affixed to a separate test tube containing lime water via a delivery tube equipped with a bulb containing anhydrous copper sulfate, as depicted in the diagram.
- During the experimental process, the organic compound undergoes oxidation, resulting in the conversion of carbon into carbon dioxide and hydrogen into water.
- The presence of carbon dioxide can be identified in lime water through the observation of a milky appearance, while in water, it can be detected using anhydrous copper sulfate, which undergoes a color change from white to blue.
C + 2 CuO [when heated Δ]→ 2 Cu + CO2
2 H + CuO [when heated Δ]→ H2O + Cu
CO2 + Ca(OH)2 [when heated Δ]→ CaCO3↓ + H2O
white ppt.
5 H2O + CuSO4 [when heatedΔ]→ CuSO4.5H2O
white blue
Detection of Oxygen
At present, a definitive means of ascertaining the presence of oxygen in organic compounds remains elusive. However, the existence of oxygen can be deduced by employing various methodologies. The subsequent enumeration comprises a selection of indirect methodologies that can be employed for the purpose of oxygen detection:
- The total percentage of all constituent elements in a compound is subtracted from 100. If the resulting value is less than 100, the difference represents the proportion of oxygen present in the compound. The detection of oxygen in organic compounds is typically carried out in a customary manner.
- The detection of oxygen-containing functional groups implies the existence of oxygen within an organic compound. Several functional groups containing oxygen can be identified, including -OH, -CHO, -CO, -COOH, -COO-, -CONH2, -NO2, and -COX, among others.
- When an organic compound that contains oxygen is heated in a nitrogen atmosphere without any chemical reactions, water droplets form on the cooler part of the tube. This shows that oxygen is present. Nevertheless, a negative test does not necessarily imply the absence of oxygen. The test in question does not provide a definitive means of detecting the presence of oxygen.
Detection of Foreign Elements
The elements that are infrequently encountered in organic compounds, such as nitrogen (N), sulfur (S), and halogens (X), are commonly referred to as exogenous elements. The bonding between these elements occurs via covalent bonds within organic compounds, which results in their inability to ionize for detection. This is due to the fact that covalent bonds possess greater strength compared to ionic and co-ordinate bonds.
- The initial stage entails the conversion of non-reactive elements into ionic compounds by fusing organic molecules with sodium metal, as they exhibit limited reactivity towards ionic reagents. Following this, a chemical examination is performed in order to ascertain and validate the existence of the particular elements under consideration.
- The sodium fusion test, which aimed to identify the presence of extraneous elements, was carried out in 1843 by J.L. Lassaigne, a renowned scientist from France. In the present experimental protocol, a little amount of an organic molecule is vigorously mixed with a recently cut slice of sodium metal inside an ignition tube.
- The ignition procedure is executed until the tube attains a condition of elevated temperature, resulting in its emission of red luminescence. Following this, the combusted substances, along with the tube used for ignition, are submerged into a receptacle containing 10 milliliters of purified water within a mortar. Subsequently, the combination is subjected to the process of pulverization by the utilization of a pestle.
- Furthermore, the ignition tube experiences compression. Following this, the entire substance undergoes filtration to create a conclusive solution known as sodium extract or Lassaigne’s solution.
- The alkaline quality of the sodium extract is attributed to the reaction between an excess of sodium and water, which leads to the production of Sodium Hydroxide (NaOH). The sodium extract consists of ionic molecules originating from external components that contain sodium.
- For instance, the halogens N, S, and X undergo conversion into sodium derivatives CN–, S—, and X–, respectively, which can be identified through distinct chemical assays. The chemical process of sodium fusion is outlined as follows:
fusion
Na + C + N → NaCN
fusion
2Na + S → Na2S
fusion
Na + X → NaX (X = Cl-,Br- or I-)Test For The Detection of N, S, and X (Halogens)
Detection of Nitrogen
- A volume of approximately 2 mL of concentrated sodium extract is introduced into a test tube and subjected to boiling in the presence of freshly prepared ferrous sulfate solution.
- The addition of a small amount of sodium hydroxide can increase the inherent alkalinity of sodium extract.
- The contents of the test tube are subjected to a cooling process, after which a small quantity of ferric chloride solution is introduced, along with an abundant amount of concentrated hydrochloric acid.
- The occurrence of Prussian blue or green pigmentation serves as evidence for the existence of nitrogen within the organic compound being examined.
- In this procedural stage, concentrated hydrochloric acid is introduced to facilitate the dissolution of surplus ferrous hydroxide. This is necessary as the presence of excess ferrous hydroxide would otherwise obscure the characteristic Prussian blue color.
FeSO4 + 2 NaOH → Fe(OH)2 + Na2SO4 Fe(OH)2 + 2 NaCN → Fe(CN)2 + 2 NaOH Fe(CN)2 + 4 NaCN → Na4[Fe9CN)6]
sodium ferrocyanide 3 Na4[Fe(CN)6] + 4 FeCl3 → Fe4[Fe(CN)6]3 + 12 NaCl
ferric ferrocyanide (Prussian blue) Fe(OH)2 + 2 HCl → FeCl2 + 2 H2O If sulfur is present along with nitrogen in the organic compound under examination, it is possible for a blood-red color to appear during the test mentioned earlier.
Na + C + S + N → NaCNS
sodium sulfocyanide (blood red) 3 NaCNS + FeCl3 → Fe(CNS)3 + 3 NaCl
ferric sulfocyanide (blood red)At times, the absence of a blood-red hue in a sample, despite the presence of nitrogen and sulfur, can be attributed to the excessive presence of sodium. In such cases, sodium causes the decomposition of NaCNS into Na2S and NaCN, thereby yielding distinct tests for each compound.
2 Na + NaCNS → Na2S + NaCNDetection of Sulfur
Multiple methodologies can be utilized to ascertain the presence of sulfur in an organic component, employing a sodium extract.
- A tiny volume of freshly made sodium nitroprusside solution is added to a test tube holding 2 mL of sodium extract.
- The presence of a deep violet color indicates the presence of sulfur in the organic molecule undergoing investigation.
Na2S + Na2[Fe(CN)5NO] → Na4[Fe(CN)5NOS]
sodium nitroprusside sodium sulfonitroprusside (deep violet)The detection of sulfur can also be achieved by performing a reaction involving 2 mL of sodium extract, acetic acid, and a small amount of lead acetate solution. The observed outcome of this reaction is the formation of a black precipitate.
Na2S + Pb(CH3COO)2 → PbS ↓ + 2 CH3COONa
lead acetate lead sulfide (black ppt.) Detection of Halogens
Halogens, which are commonly found in organic compounds, can consist of chlorine (Cl), bromine (Br), or iodine (I). Certain compounds may also incorporate the element F, which exhibits high reactivity and electronegativity. It is worth noting that this element has not been explicitly addressed in the preceding discussion.
- A sodium extract is utilized for the detection of organic compounds containing halogens.
- In a test tube, 2 mL of sodium extract is heated with a small amount of dilute nitric acid in order to remove any sulfides, cyanides, or phosphides that may be present in the compound being tested.
- If an organic compound contains halogens, as well as elements such as sulfur (S), nitrogen (N), phosphorus (P), and others, various outcomes may arise.
- Next, the clear solution is subjected to a reaction with a solution of silver nitrate. The resulting precipitate of silver halide in ammonia solution is carefully observed for changes in color and solubility.
AgNO3 + X- → AgX ↓ + NO-3
ppt.Silver chloride is a white precipitate characterized by a curdy appearance. It demonstrates full solubility in ammonia solutions. The presence of a white precipitate formation upon the addition of silver nitrate solution indicates the possible presence of chlorine within the organic compound.
NaCl + AgNO3 → AgCl ↓ + NaNO3
white ppt. AgCl + 2 Na4OH → Ag(NH3)2Cl + 2 H2O
solution (diammine silver chloride) On the contrary, silver bromide demonstrates a subtle yellow sediment, showcasing restricted solubility in ammonia solutions. This observation indicates the presence of bromine in an organic compound.
NaBr + AgNO3 → AgBr ↓ + NaNO3
pale yellow ppt. AgBr + 2 NH4OH → Ag(NH3)2Br + 2 H2O
sodium (diammine silver bromide)Similarly, the introduction of iodide ions leads to the creation of a yellow precipitate upon combination with a solution of silver nitrate. The observed precipitate demonstrates full insolubility upon exposure to an ammonia solution. Hence, if the sodium extract of the organic compound produces this observation, it can be inferred that the presence of iodine has been verified.
NaI + AgNO3 → AgI ↓ + NaNO3
yellow ppt.In this particular scenario, the coexistence of S and N elements within an organic compound, in conjunction with halogens, may result in potential interference during the halogen detection process.
The interference arises as a result of the chemical reaction between sulfur and halogens, leading to the formation of silver sulfide. Similarly, the reaction between nitrogen and halogens results in the formation of silver cyanide.
Before introducing the silver nitrate solution, the utilization of nitric acid on the sodium extract effectively eliminates the presence of hydrogen sulfide and hydrogen cyanide gases. These gases contain sulfur and nitrogen components.
Na2S + 2 AgNO3 → Ag2S ↓ + 2 NaNO3
silver sulfide (black ppt.) NaCN + AgNO3 → AgCN ↓ + NaNO3
silver cyanide (white ppt.) Na2S + 2 HNO3 → H2S ↑ + 2 NaNO3 NaCN + HNO3 → HCN ↑ + NANO3Video on Qualitative Analysis of Organic Compounds
Frequently Asked Questions (FAQ)
What is the methodology employed for the estimation of carbon and hydrogen?
Liebig’s combustion method is employed for the determination of carbon and hydrogen content. The hydrogen species undergoes oxidation to form water, while the carbon species undergoes oxidation to produce carbon dioxide.
What is the methodology employed for nitrogen estimation?
The Dumas method is employed for the determination of hydrogen. The liberation of nitrogen gas in its unbound state serves as a confirmation of the existence of nitrogen.
Why is organic analysis important?
The utilization of organic analysis facilitates the elucidation of the composition, arrangement, and characteristics of unfamiliar compounds. The identification of functional groupings can be achieved by employing both qualitative and quantitative methods.
What is the Lassaigne test?
Lassaigne’s test is a test developed by J L Lassaigne, for the detection of elements such as Nitrogen(N), Sulfur(S), Chlorine (Cl), Bromine (Br), and Iodine (I).
The Lassaigne test is employed for the identification of sulfur, halogen, and nitrogen elements inside organic molecules. To do the experiment, a fusion tube should be utilized, wherein the organic chemical is introduced alongside a little sodium fragment. Through this process, sodium effectively transforms all the accessible metals into their respective ionic compounds.
References
- https://byjus.com/jee/qualitative-analysis-of-organic-compounds/
- https://www.toppr.com/guides/chemistry/organic-chemistry/qualitative-analysis-of-organic-compounds/
- https://www.vedantu.com/iit-jee/qualitative-analysis-of-organic-compounds
- https://chem.libretexts.org/Ancillary_Materials/Laboratory_Experiments/Wet_Lab_Experiments/Organic_Chemistry_Labs/Intermediate_Chemical_Experimentation/02%3A_Qualitative_Organic_Analysis/2.01%3A_New_Page
- https://www.studysmarter.co.uk/explanations/chemistry/organic-chemistry/organic-analysis/
- Lawrie Ryan, Cambridge International AS and A Level Chemistry Coursebook, 2014
- https://www.geeksforgeeks.org/qualitative-analysis-of-organic-compounds/