Carbon, with the atomic number 6 and the symbol C, is found in Periodic Table Group 14. Carbon is a nonmetal that can be found in nature in the forms of graphite, diamond, or fullerenes. It has four electrons available to form covalent connections, making it tetravalent. Carbon is the world’s sixth most prevalent element. Carbon, although being extensively dispersed in nature, is not very abundant—it accounts for only approximately 0.025 percent of the Earth’s crust—yet it forms more compounds than all other elements combined.
Interesting Science Videos
History of Carbon
Carbon was found in prehistory and was known to the oldest human civilizations in the forms of soot and charcoal. Carbon is derived from the Latin carbo, which means coal or charcoal.
- René Antoine Ferchault de Réaumur proved in 1722 that iron could be turned into steel by the absorption of some material, now known to be carbon.
- Antoine Lavoisier demonstrated in 1772 that diamonds are a kind of carbon by burning samples of charcoal and diamond and discovering that none created any water and both emitted the same amount of carbon dioxide per gram.
- Carl Wilhelm Scheele demonstrated in 1779 that graphite, previously assumed to be a type of lead, was really identical with charcoal but with a little admixture of iron, and that when oxidized with nitric acid, it produced carbon dioxide.
- The French scientists Claude Louis Berthollet, Gaspard Monge, and C. A. Vandermonde verified that graphite was primarily carbon in 1786 by oxidizing it in oxygen, just like Lavoisier had done with diamond.
- Some iron was left over, which the French scientists believed was required for the graphite structure. In their paper, they proposed the term carbone (Latin carbonum) for the element in graphite that emitted a gas when burned.
- Carbon was then recognized as an element by Antoine Lavoisier’s in 1789.
Occurrence of Carbon
After hydrogen, helium, and oxygen, carbon is the fourth most abundant chemical element in the observable universe by mass. Carbon is a common element of all known life due to its abundance, remarkable diversity of organic compounds, and extraordinary ability to form polymers at typical Earth temperatures. It is the second most prevalent element in the human body by mass (approximately 18.5%) after oxygen.
Carbon’s abundance, unique range of organic compounds, and rare ability to form polymers at typical Earth temperatures allow it to act as a common ingredient of all known life. It is the second most prevalent element in the human body by mass (approximately 18.5%). Carbon is a component (approximately 12% by mass) in extremely large amounts of carbonate rock (limestone, dolomite, marble and so on). Coal is exceptionally rich in carbon (anthracite contains 92-98%) and is the most important commercial source of mineral charcoal, accounting for 4,000 gigatonnes or 80% of fossil fuel use.
Isotopes of Carbon
Carbon isotopes are atomic nuclei that contain six protons and a number of neutrons (varying from 2 to 16). Carbon has two naturally occurring stable isotopes- 12C and 13C.
- Carbon-12 (12C) accounts for 98.93% of all carbon on Earth
- Carbon-13 (13C) accounts for the remaining 1.07%.
Carbon-14 (14C) is a naturally occurring radioisotope produced by the interaction of nitrogen with cosmic rays in the high atmosphere (lower stratosphere and upper troposphere). It is created by the neutron-proton reaction on nitrogen caused by thermal neutrons emitted by cosmic radiation. 14C is almost non-existent in old rocks. The quantity of 14C in the atmosphere and live creatures is nearly constant, but it declines predictably after death. This concept is applied in radiocarbon dating. The C-14 and C-12 ratio is commonly employed in radiocarbon dating for determining the age of plants and animals.
Allotropes of Carbon
Atomic carbon has a relatively limited lifetime and is stabilized by a variety of multi-atomic formations known as allotropes. Diamond and graphite are two prevalent allotropic carbon forms. Additional allotropes include:
- β-graphite
- Amorphous carbon
- Lonsdaleite, also known as hexagonal diamond
- Chaoite (extremely uncommon mineral) (very rare mineral)
- Fullerenes (Buckyballs) (Buckyballs)
Graphite is one of the most prevalent and well-known carbon allotropes. It may be found in pencils. Most people call it pencil lead, although despite the name, it has nothing to do with lead. Graphite is entirely composed of carbon atoms. Within graphite, each carbon atom uses its lone electron to establish three covalent connections with nearby carbon atoms, resulting in two-dimensional sheets.
Diamond is another another carbon allotrope. Diamond, unlike graphite, is created by carbon atoms forming a pyramid-like three-dimensional structure. Inside a diamond, each carbon atom establishes four covalent connections with adjacent atoms. As a result, unlike graphite’s sheet-like structure, all atoms are closely bonded.
Buckminsterfullerene (C60) is another carbon allotrope. Fullerene has a cage-like structure, which gives it the appearance of a football.
Elemental Properties
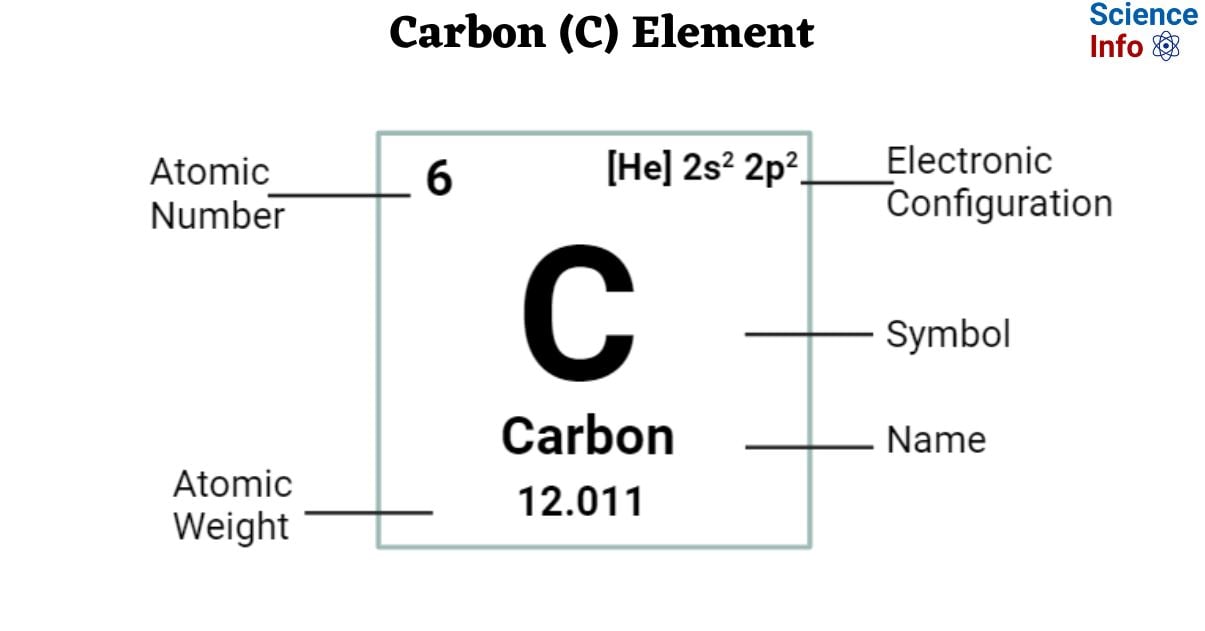
| Electronic Configuration | [He] 2s2 2p2 |
| Atomic Number | 6 |
| Atomic Weight | 12.011 |
| Group, Period, and Block | 14, 2, p-block |
| Empirical Atomic Radius | 70 pm |
| Covalent radius | 76(1) [sp3], 73(2) [sp2], 69(1) [sp] pm |
| Van der Waals radius | 0.091 nm |
| Electrons: | 6 |
| Protons | 6 |
| Neutrons in the most abundant isotope: | 6 |
| Electron shells | 2, 4 |
Physical Properties of Carbon
- The symbol C represents this chemical element.
- It bears the atomic number 6 because its nucleus contains 6 protons.
- Carbon is both nonmetallic and tetravalent.
- It has three naturally occurring isotopes ( 12C, 13C – stable, and 14C – radioactive)
- It is available in a variety of forms. It appears in several allotropes and other forms. Diamond and graphite are two materials that have different characteristics.
- Carbon may exist in an amorphous form as well. Several amorphous allotropes, such as glassy carbon, soot, or carbon black, contain enough structure to be classified as not genuinely amorphous. Although crystalline nanotubes have been detected, the vast majority of them are amorphous.
- Carbon has the greatest melting/sublimation point of any element and the maximum thermal conductivity of any element in the form of diamond.
- The chemical element has the ability to make bonds with other elements. To establish a covalent bond, carbon generates four electrons.
| Physical State | Solid |
| Element Classification | Non metal |
| Melting Point | Sublimes at 3825°C, 6917°F, 4098 K |
| Boiling Point | Sublimes at 3825°C, 6917°F, 4098 K |
| Density | 3.513 (diamond); 2.2 (graphite) |
| Triple Point | 4600 K, 10,800 kPa |
| Heat of Fusion | graphite: 117 kJ/mol |
| Electronegativity | 2.55 (Pauling Scale) 2.544 (Allen Scale) |
| Electron Affinity | 1.263 eV |
Chemical Properties of Carbon
Carbon is used in numerous reactions throughout all fields of chemistry. Combustion reactions, in which a hydrocarbon combines with oxygen to produce carbon dioxide and water, are among the most prevalent. Ocean acidification is another significant carbon-related response. As carbon dioxide dissolves in the ocean, it interacts to generate carboxylic acids, lowering the pH. Carbon, in general, does not need a lot of work to separate. Coal, diamond, and graphite are all pure forms of carbon that exist naturally.
- Carbon is very reactive with many smaller atoms and creates stable covalent bonds.
- Even though it reacts with a large number of atoms, carbon is very moderately reactive.
- Carbon is an amphoteric element, which means it can react with both acids and bases. It is also a good electrical conductor.
- When carbon is present at standard temperature and pressure, it tends to resist oxidation and does not react with hydrochloric acid, chlorine, or any alkali metals.
- At higher temperatures, carbon tends to react with oxygen to produce carbon oxides and with metals to produce metal carbides.
- Carbon monoxide and methane are two prominent compounds of carbon. Methane has one of the highest warming potentials per molecule of any greenhouse gas. Carbon monoxide is a colorless, odorless gas that may kill humans. Nevertheless, carbon monoxide may also be used as a ligand in a variety of inorganic processes.
- Carbon is the element that gives rise to numerous significant ions in chemistry, including the carbonate ion CO3-2, the bicarbonate ion HCO3–, and the carbide ion C-4.
- As washing soda, sodium carbonate is utilized. Except for alkali metal carbonates, most carbonates are insoluble in water. Carbonates are useful in chemistry because they may be mixed with an acid to generate any other element’s salt. In solution, soluble carbonates such as sodium carbonate and potassium carbonate are basic.
Uses and Applications of Carbon
It is a free element with a wide range of applications.
- Diamond ornamentation in jewelry or black fume pigment in automotive rims and printer ink.
- Another kind of carbon is graphite, which has been used in high-temperature crucibles, arc lamp electrodes, dry cells, and pencil tips.
- Vegetal carbon is another amorphous form of carbon that is employed as a bleaching agent and a gas absorbent.
- To carbonate beverages, they utilize carbon dioxide and a fire extinguisher.
- In several metallurgical processes, carbon monoxide is utilized as a reduction agent.
- Carbon tetrachloride and carbon disulphide are commonly used industrial solvents. In cooling systems, freon is employed.
- Calcium carbide is used to make acetylene; it is also used to weld and cut metals, as well as to make various organic compounds.
- Other metallic carbides are useful as heat-resistant materials and metal cutters.
- Carbon is essential to every known biological system, and life would not exist without it.
- Apart than food and wood, it may be accessible in hydrocarbon forms such as methane gas, crude oil, and fossil fuel.
- Carbon fibers have several applications due to its characteristics as a lightweight, robust, and long-lasting material.
- These fibers have the potential to be employed in the manufacture of fishing rods, tennis rackets, and even rockets and airplanes.
Biological Role of Carbon
- Carbon is a minor component of the Earth’s crust. Carbon is abundant in the form of coal and organic molecules.
- They are composed of natural gas, petroleum, as well as plant and animal tissue. Carbon is required for photosynthesis to take place.
- It is a natural chemical reaction sequence in which the carbon cycle is converted to generate atmospheric carbon dioxide and carbohydrates.
- Carbon is contained in organic materials such as DNA, proteins, and other parts of the human body. Carbon is also an important tool for connecting the atoms together.
- Carbon is found in all living things, including animals, people, and plants, as well as rocks. Yet, carbon accounts for just 0.025% of the Earth’s crust.
- Carbon is present in all living forms and produces a wide range of compounds, including fats and sugars.
- All living things are built on a carbon base.
- The human body has almost 19% carbon (by weight).
- It may also be inorganic, combining with oxygen and other molecules to form significant portions of the non-living world, such as rocks and minerals.
Read Also: Beryllium
Health and Environmental Effects of Carbon
Health Effects
Elemental carbon is extremely poisonous. The health hazard statistics reported here is based on carbon black exposure rather than elemental carbon.
- Prolonged inhalation of carbon black may cause temporary or permanent lung and heart damage.
- Workers involved in the manufacture of carbon black have been reported to have pneumoconiosis.
- Skin disorders such as hair follicle irritation and mouth mucosal lesions have also been observed as a result of skin exposure.
- Certain basic carbon compounds, such as carbon monoxide (CO) or cyanide, may be extremely dangerous (CN–).
- Carbon 14 is one of the radionuclides used in nuclear bomb atmospheric testing, which began in 1945 with a US test and concluded in 1980 with a Chinese test. It is one among the long-lived radionuclides that have caused and will continue to cause increasing cancer risk in the next decades and millennia. It can also cross the placenta and become organically bonded in growing cells, putting fetuses at risk.
- The majority of what we consume is composed of carbon molecules, resulting in a total carbon intake of 300 g per day. Digestion involves breaking these substances down into molecules that can be adsorbed to the stomach or gut wall. The blood transports them to areas where they are used or oxidized to release the energy they contain.
Environmental Effects
- Carbon is normally non-toxic to life on Earth; nonetheless, carbon nanoparticles are lethal to Drosophila.
- In the presence of air at high temperatures, carbon may burn furiously and brightly. When exposed to air, large accumulations of coal that have been inert for hundreds of millions of years in the absence of oxygen may spontaneously combust in coal mine waste tips, ship cargo holds and coal bunkers, and storage dumps.
- Polycyclic aromatic hydrocarbons can come from natural sources or be generated by a variety of human activities. Aluminum factories, coke plants, iron and steel industries, ferroalloy plants and foundries, vehicles and other petroleum-burning engines (including airplanes), and garbage disposal by burning are examples of these.
- The study of automotive exhaust emissions is critical for assessing the consequences of coal conversion since they can be a major source of carcinogenic chemicals in the atmosphere.
Toxicity, Safety, and Precautions

Major routes of entry: inhalation, eye and skin contact.
Exposure to the eyes: It can occur through direct contact with airborne particulates or through contact with contaminated hands or clothing. Particulate can cause irritation or mechanical harm to the eyes.
Skin: Particle that becomes stuck under the skin can cause hypersensitivity and skin sores.
Inhalation: May irritate the mucous membranes in the nose, throat, lungs, and other areas.
Safety Measures
- After making eye contact, immediately rinse your eyes thoroughly with water for at least 15 minutes while occasionally elevating your top and lower eyelids. Seek medical help right away.
- Remove all contaminated clothing, wash it, and then dry it. Wash skin cuts and wounds thoroughly to get rid of any granular material. For wounds that cannot be properly cleaned, need medical help. Before starting work, treat skin cuts and wounds using common first aid procedures such washing, disinfecting, and covering to stop infection and contamination. If you are constantly irritated, get medical attention. It is necessary to remove any material unintentionally implanted or lodged under the skin.
- Breathing discomfort brought on by particle inhalation necessitates a swift transfer to fresh air. If you have breathing problems, you could need oxygen. Use artificial respiration if breathing has ceased, then seek medical attention.
- Most significant acute and delayed signs and symptoms: could result in an allergic skin reaction. allergic respiratory response could occur. Chronic consequences may result from prolonged exposure.
Precautions
Personal precautions: Wet carbon black produces slippery walking surfaces. Avoid dust formation. Wear appropriate personal protective equipment and respiratory protection.
For emergency responders: Use personal protective equipment.
Environmental precautions: Carbon black poses no significant environmental hazards.
Contain spilled product on land, if possible. As a matter of good practice, minimize contamination of sewage water, soil, groundwater, drainage systems, or bodies of water. Prevent further leakage or spillage if safe to do so.
Handling and Storage
- Prevent the production of dust. Do not inhale dust. Give adequate local exhaust to reduce dust buildup. Never use pressurized air.
- Take precautions to avoid static discharges. Offer sufficient safeguards, such as electrical grounding and bonding or inert atmospheres. In some scenarios, grounding of equipment and conveying systems may be necessary.
- The elimination of potential ignition sources in close proximity to carbon black dust; good housekeeping to avoid dust accumulations on all surfaces; and appropriate exhaust ventilation design and maintenance to control airborne dust levels to below the applicable occupational exposure limit are all examples of safe work practices.
- Whenever hot work is necessary, the surrounding region must be free of carbon black dust.
- Store in a dry, cool, and well-ventilated area. Keep away from sources of ignition, heat, and powerful oxidizers.
- Test for enough oxygen, combustible gases, and possibly harmful air pollutants before entering containers and tight spaces containing carbon black. Let no dust to build on surfaces.
References
- https://pubchem.ncbi.nlm.nih.gov/element/Carbon#section=Electronegativity
- Mary Elvira Weeks, The discovery of the elements. I. Elements known to the ancient world., J. Chem. Educ., 1932, 9 (1), p4
- Jessica Elzea Kogel, Industrial minerals & rocks: commodities, markets, and uses., (2006) p507. SME.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- Amanda S. Barnard, The diamond formula: diamond synthesis–a gemological perspective., (2000) p3. Butterworth-Heinemann
- Robert M. Hazen, The diamond makers., (1999) p145. Cambridge University Press.
- Jonathan W. Steed, Jerry L. Atwood, Supramolecular Chemistry., (2009) p423. Wiley.
- The Environmental Significance of Coal-derived Carbon Compounds. https://doi.org/10.1016/B978-0-08-028734-8.50015-2
- https://www.geeksforgeeks.org/carbon-definition-properties-occurrence-applications/
- https://www.rsc.org/periodic-table/element/6/carbon
- https://absolutecarbonfilters.co.uk/2021/01/28/6-interesting-facts-about-carbon/
