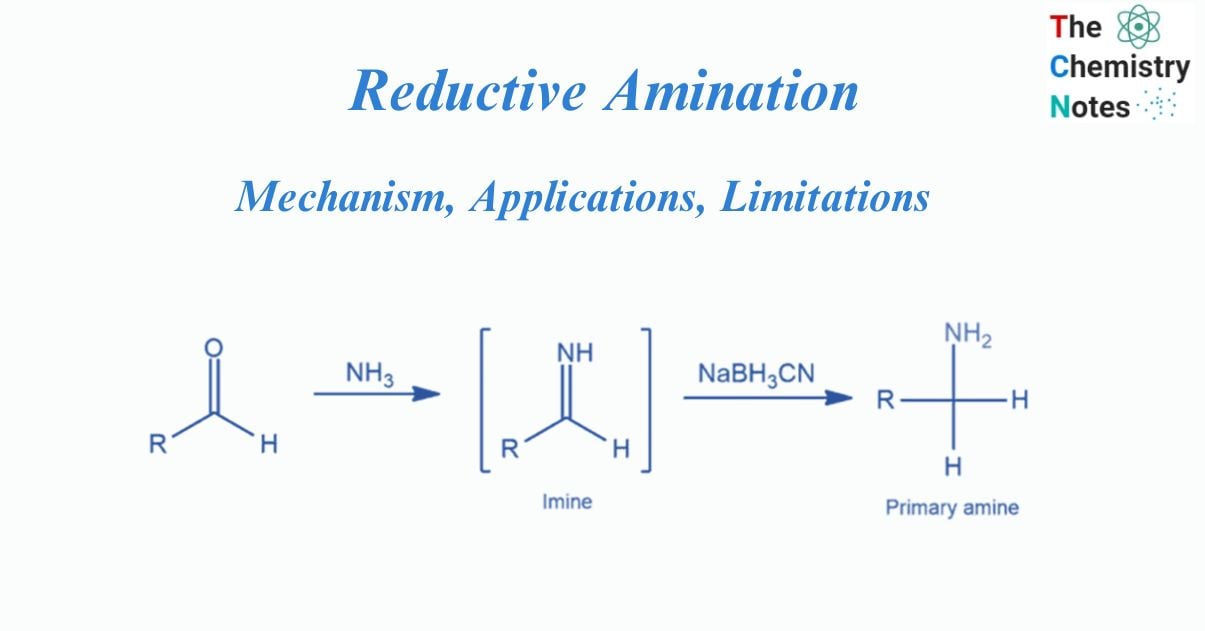
Reductive amination is the process of conversion of a carbonyl group to an amine through an imine ion intermediate. It is a chemical reaction that converts aldehyde and ketones into 1°, 2° and 3° amine.
It is an excellent method to generate the imine of an amine with a suitable aldehyde or ketone, and then reduce the imine to an amine. It avoids the issue of repeated alkylations.
Sodium cyanoborohydride (NaBH3CN) is a common reducing agent because it can selectively reduce imines in the presence of aldehydes. The hydride reagent is a sodium borohydride (NaBH4) derivative produced by substituting one H atom with CN. Because it would reduce the ketone before it could be turned into an imine, sodium borohydride cannot be employed. Sodium cyanoborohydride, on the other hand, does not react with ketones but reduces an iminium ion.
Many other reducing agents Like NaBH4, and NaBH(OAc)3, can also be utilized.
Interesting Science Videos
What is Reductive Amination?
The process of converting aldehyde and ketone into primary, secondary, and tertiary amines is known as reductive amination.
In order to produce a new alkylated amine directly, the carbonyl molecule is exposed to an amine that has at least one nitrogen-hydrogen bond and a reducing agent such as NaBH3CN in this process. The carbonyl carbon of the aldehyde or ketone forms a new carbon-nitrogen bond. Reductive amination substitutes a carbon-oxygen double bond with carbon-hydrogen and carbon-nitrogen bonds.
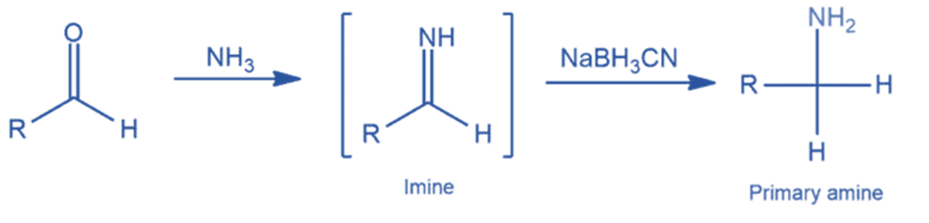
Mechanism of Reductive amination
An imine is formed by the nucleophilic attack of an amine (ammonia, primary, or secondary amine) on the carbonyl group. The resultant imine will then be protonated under acidic environments to yield an iminium ion (its conjugate acid). Finally, in the presence of a reducing agent, the iminium ion will be reduced to provide a new amine.
Limitation of Reductive Amination
- Reductive amination does not generate bonds between nitrogen and aromatic rings.
Applications of Reductive Amination
- One advantage of reductive amination is that it avoids the problem of over-alkylation that occurs with direct alkylation of amines with alkyl halides.
- Reductive amination of aldehydes and ketones is regarded as one of the most essential methods of producing secondary and tertiary amines in modern chemical synthesis.
- As chiral amines are present in many of the world’s most significant medications, asymmetric reductive aminations are among the most essential reactions in the manufacture of active pharmaceuticals.
References
- Morrison R. T. & Boyd R. N. (1983). Organic chemistry (4th ed.). Allyn and Bacon.
- Smith M. & March J. (2001). March’s Advanced Organic Chemistry: Reactions Mechanisms and Structure (5th ed.). Wiley.
- Ghosh, S.K., Advanced General Organic Chemistry, Second Edition, New Central Book Agency Pvt. Ltd., Kolkatta, 2007.
- Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and Company Ltd, New Delhi, 1992. production of polyesters, polyurethanes, and alkaline resins.
