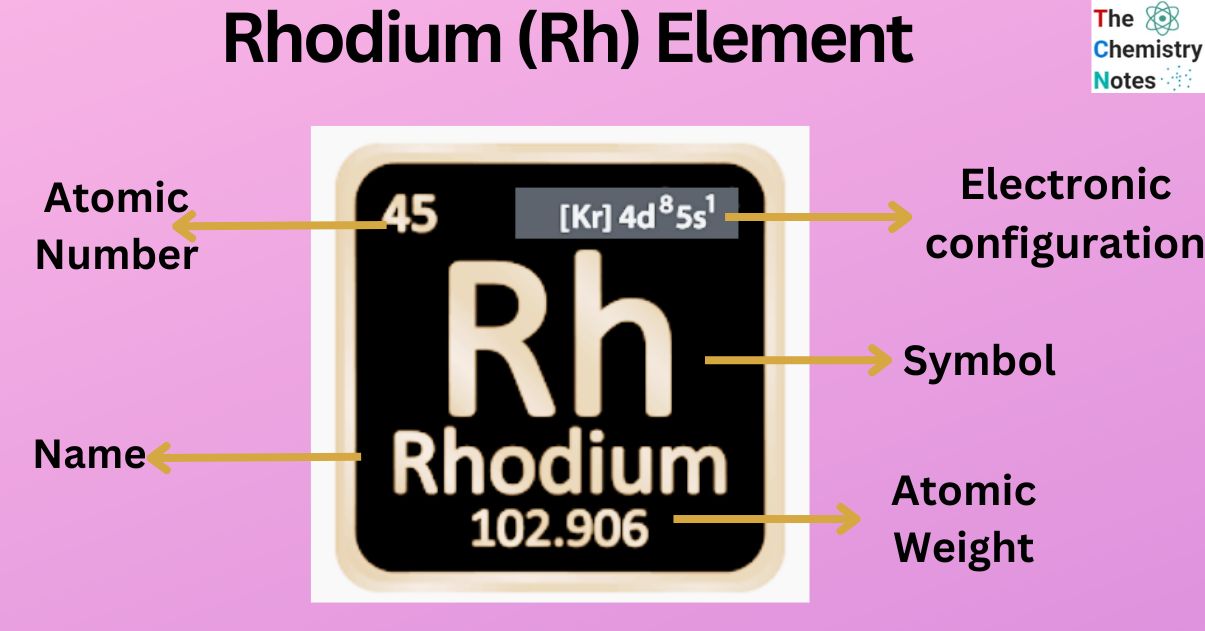
Rhodium is a metallic element with the atomic number 45 and is represented by the symbol ‘Rh’ in the periodic table. It is classified as a transition metal and belongs to the d-block of group 9 of the periodic table. It is one of the rarest and valuable metal that belongs to the platinum group of the periodic table.
Rhodium is a relatively rare metal that is hard, lustrous, and silvery-white at room temperature. It is the most expensive precious metal.
Interesting Science Videos
History of Rhodium
- In the year 1803, an English chemist, William Hyde Wollaston, discovered rhodium. He had also discovered palladium earlier.
- William Hyde Wollaston had obtained rhodium from a sample of platinum ore that was obtained from South America.
- Wollaston removed the platinum and palladium from the sample he had obtained. After the procedure, he was left with a dark red powder. It was identified as sodium rhodium chloride (Na3RhCl6.12H2O).
- He obtained rhodium from the powder (sodium rhodium chloride) by treating it with hydrogen gas (H2).
- Rhodium got its name from the Greek word “rodhon” meaning the rose colored.
Occurrence Of Rhodium
- Rhodium is found in ores combined with other metals like palladium, silver, platinum, and gold, making its industrial extraction challenging.
- Rhodium is typically obtained as a byproduct of mining and refining platinum which usually exists with mines of platinum.
- Major mines of rhodium are located in the river sands of the Ural Mountains, South Africa, North America, and South America.
- Rhodium is also obtained as a byproduct of the nickel mining operation in the Sudbury region of Ontario, Canada.
- Although the quantity at Sudbury is not high, the huge volume of nickel ore processed makes rhodium extraction economically feasible.
- Rhodium has 24 isotopes whose half-lives are known, with mass numbers ranging from 94 to 117.
Isotopes Of Rhodium
Rhodium has only one naturally occurring stable isotope.
Naturally occurring isotope of Rhodium
| Isotope | Natural abundance (atom %) |
|---|---|
| 103Rh | 100 |
Elemental Properties of Rhodium
| Electronic Configuration | [Kr] 4d8 5s1 |
| Atomic Number | 45 |
| Atomic Weight | 102.9055 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | 9, 5, d-block |
| Density | 12.41 g.cm -3 at 20 °C |
| Van der Waals radius | 0.135 nm |
| Electron shells | 2, 8, 18, 16, 1 |
| Electrons | 45 |
| Protons | 45 |
| Neutrons in most abundant isotope | 58 |
Physical Properties of Rhodium
- Rhodium has an atomic number of 45 and is a silvery-white metal. It has a melting point of 1963°C (3565°F) and a boiling point of 3695°C (6683°F).
- Ruthenium has a solid phase density of 12.41 gm/cm3 and a liquid or molten phase density of 10.70 gm/cm3.
- Rhodium serves as an excellent electrical conductor. Because electrons in ruthenium are free to move around, they can carry electrical charge from one end to the other. It is the most electrically conductive of the platinum groups.
- It is an effective thermal conductor as well. Heat causes a metal’s particles to vibrate more rapidly and move around more swiftly. Energy is transferred from one particle to another as they come into contact.
- It is highly corrosion-resistant.
| Color/physical appearance | Lustrous, silvery, white |
| Melting point/freezing point | 1963°C, 3565°F, 2236 K |
| Boiling point | 3695°C, 6683°F, 3968 K |
| Density | 12.41 g cm-3 at 20° |
| Malleability | Yes |
| Ductility | Yes |
| Electronegativity | 2.28 (Pauling Scale) |
Chemical Reaction of Rhodium
- Reaction of Rhodium with air
To a significant extent, rhodium is resistant to reaction with air. Rhodium metal transforms into rhodium (III) oxide, Rh2O3, when heated with oxygen at 600 degrees Celsius.
4Rh(s) + 3O2(g) → 2Rh2O3(s) [dark grey]
- Reaction of Rhodium with water
Under typical circumstances, water and rhodium do not react.
- Reaction of Rhodium with the halogens
Rhodium(VI) fluoride, often known as RhF6, is created when fluorine gas and metallic rhodium immediately react. With care, it is possible to heat this substance to produce [RhF5]4, a dark red tetrameric form of rhodium(V) fluoride.
Rh (s) + 3F2(g) → RhF6(s) [black]
Rhodium(III) can directly react with halogens to generate the trihalides rhodium(III) fluoride, rhodium(III) chloride, and rhodium(III) bromide, i.e. IrF3, IrCl3, and IrBr3, respectively, when the reaction is carried out in anhydrous (dry) conditions.
2Rh (s) + 3F2(g) → 2RhF3(s) [red]
2Rh (s) + 3Cl2 (g) → 2RhCl3 (s) [red]
2Rh (s) + 3Br2 (g) → 2RhBr3 (s) [red-brown]
- Reaction of Rhodium with acids
In particular, aqua regia, a solution of hydrochloric acid (HCl) and nitric acid (HNO3) famed for its capacity to dissolve gold metal, has no effect on the reactivity of rhodium metal with acids.
Uses of Rhodium
- As a Catalytic Converter
Rhodium is mostly utilized in the production of catalytic converters for gasoline-powered vehicles. In fact, this application accounts for little more than three-quarters of global rhodium demand. Catalytic converters, in essence, transform toxic gases in exhaust (particularly nitrogen oxides) into gases that are less destructive to the environment and our health, resulting in less harmful emissions. Rhodium, frequently in conjunction with palladium and/or platinum, achieves this via lowering nitrogen oxide levels in exhaust gases.
- As a Catalyst
Rhodium is also utilized as a catalyst in the chemical industry in the production of nitric acid, acetic acid, and hydrogenation processes. 5-7% of rhodium is used to make catalysts for the chemical industry. Rhodium-containing catalysts, for example, aid in the creation of raw materials used in the manufacture of fertilizers and explosives.
- Alloys
Rhodium is frequently alloyed with platinum and iridium to create an oxidation-resistant metal capable of withstanding high temperatures. Rhodium is commonly used as an alloying agent to strengthen platinum and palladium.
For aircraft turbine engines, it is also alloyed with platinum.
These alloys are utilized in furnace windings, pen nibs, phonograph needles, high-temperature thermocouple and resistance wires, electrodes for aviation spark plugs, bearings, and electrical connections, and many more applications.
- Glass Production
Glass manufacture accounts for another 3-6% of rhodium use. This precious metal has the ideal properties for making vessels that contain and shape molten glass. It is also utilized in the manufacture of glass fiber and liquid crystal displays (LCDs).
- Jewelry
Rhodium’s brilliant brilliance and resistance to scratching and tarnishing make it ideal for jewelry plating. Rhodium is also utilized as a finish for mirrors, optical instruments, electrical components, high-heat manufacturing and lab equipment, electrodes for aircraft spark plugs, thermocouples, sputtering targets, and other items.
Health and Environmental Effects of Rhodium
The lack of data on Rh toxicity and its effects, the widespread belief that environmental Rh levels are too low to pose a serious threat to human health, and the belief that Rh is only released in its metallic, relatively inert form in biological reactions have all prevented an adequate assessment of the risks associated with this metal’s environmental exposure.
However, recent rises in ambient levels, as well as new information on Rh concentrations in the tiniest fractions of particulate matter, have sparked much interest and debate over the potential impact of this metal on human health. Furthermore, the findings of several innovative studies that demonstrated the cytotoxic and genotoxic effects of Rh on cellular systems and the induction of immunological alterations in animal models have provided the impetus for further research into the effects of Rh on human health and a reassessment of the risk derived from exposure to the metal.
Rhodium Plating
Rhodium plating, also known as dipping or flashing, produces a long-lasting, scratch-resistant, and gleaming piece of jewelry. Jewelry designers typically employ rhodium of 0.75 to 1.0 microns over silver-colored metals such as white gold or silver.
- Electroplating is used in the majority of rhodium plating techniques. Jewelers steam or electro-clean the original piece to eliminate any dirt or pollutants that could interfere with the plating procedure. After cleaning, the jeweler applies a positive electrical charge to the object before fusing a thin layer of metal.
- Jewelers should avoid employing rhodium plating with a thickness greater than 1.0 micron because the fragile metal is prone to fracture. If the layer is too thin, the metal beneath it may tarnish. Rings, for example, require a thicker covering of rhodium than earrings or pendants.
- Electroplating is a precise operation that might take up to 90 minutes each item of jewelry. The electric current must be just correct, otherwise the plating will turn black and harm the metal beneath it.
References
- Applications of Rhodium and Ruthenium Catalysts for CO Oxidation: an Overview Subhashish Dey & Ganesh Chandra Dhal https://doi.org/10.1007/s41050-020-00023-5
- https://www.sciencedirect.com/science/article/abs/pii/0376458381900297
- 022/04/06/what-is-rhodium-used-for/
- https://chemistrytalk.org/rhodium-element/
- https://kidadl.com/facts/rhodium-facts-all-about-the-most-precious-metal-on-the-earth
- https://www.sciencedirect.com/science/book/9780128229460: https://doi.org/10.1016/B978-0-12-822946-0.00025-8

