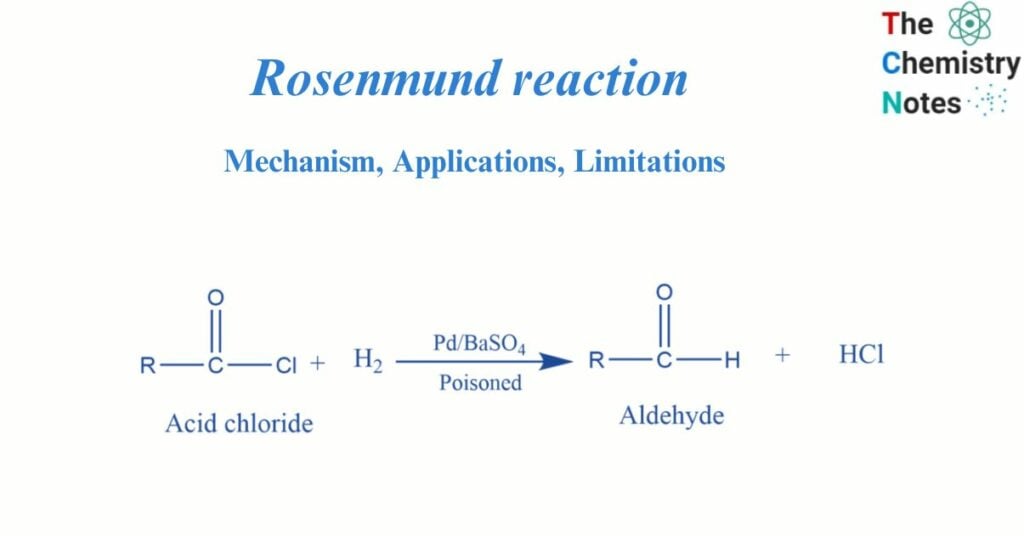
The Rosenmund reaction is a hydrogenation reaction that converts an acyl chloride to an aldehyde. To make an aldehyde from acyl chloride, the Rosenmund reduction procedure is employed. This hydrogenation procedure converts an acyl chloride to an aldehyde selectively. The reaction was named after Karl Wilhelm Rosenmund, who discovered it in 1918.
The Rosenmund reaction occurs when acid chlorides are catalytically reduced with palladium and barium sulfate in either quinoline or sulfur. Because of its low surface area, barium sulfate contributes to the reduction of palladium activity. This inhibits the over-reduction of the reaction product.
Thiourea and thioquinanthrene are two popular catalyst poisons used to limit palladium’s activity in the Rosenmund reaction. The requirement for additional palladium deactivation arises because the reaction reduces the aldehyde produced from the reduction of the acyl chloride to primary alcohol.
In the Rosenmund approach, this is an undesirable reaction because the primary alcohol interacts with the residual acyl chloride to generate an ester. Deactivation is required for the reaction since the system has to reduce the acyl chloride but not the generated aldehyde.
Interesting Science Videos
What is Rosenmund reaction?
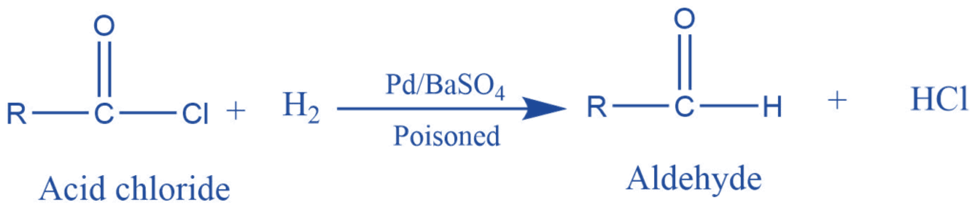
Reduction of acid chloride by hydrogenation in the presence of palladium poisoned by barium sulfate gives aldehyde. This is called the Rosenmund reaction.
The catalyst is made by reducing a solution of palladium (II) chloride in the presence of barium sulfate. This method cannot produce formaldehyde because formyl chloride is unstable at ambient temperature. Rosenmund reduction is used in the production of saturated fatty aldehyde.
Mechanism of Rosenmund reaction
When hydrogen gas is passed through the acyl chloride in the presence of a rosenmund catalyst, aldehyde, and hydrochloric acid are formed. In this reaction, barium sulfate reduces the activity of palladium and prevents over-reduction.

Applications of Rosenmund reaction
- Aldehydes are produced via the Rosenmund reduction process.
- It is used to make saturated fatty aldehydes.
- It is used in the synthesis of alkyl and aryl aldehydes.
Limitations of Rosenmund reaction
- Although this reductive process can yield several aldehydes, formaldehyde cannot be synthesized because formyl chloride is unstable at ambient temperature.
References
- Morrison R. T. & Boyd R. N. (1983). Organic chemistry (4th ed.). Allyn and Bacon.
- Smith M. & March J. (2001). March’s Advanced Organic Chemistry: Reactions Mechanisms and Structure (5th ed.). Wiley.
- Ghosh, S.K., Advanced General Organic Chemistry, Second Edition, New Central Book Agency Pvt. Ltd., Kolkatta, 2007.
- Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and Company Ltd, New Delhi, 1992. production of polyesters, polyurethanes, and alkaline resins.
