Rubidium is a metallic element with the atomic number 37 and is represented by the symbol ‘Rb’ in the periodic table. It belongs to the s-block of a group of the periodic table. It is the first alkali metal in the group with a density greater than that of water. It is a very soft, ductile, silvery-white metal.
Rubidium is the twenty-third most prevalent element in the Earth’s crust, roughly equal to zinc and slightly more common than copper. It can be found in the minerals leucite, pollucite, carnallite, and zinnwaldite, which contain up to 1% rubidium oxide.
Interesting Science Videos
History of Rubidium
- Robert Bunsen (a German chemist) and Gustav Kirchhoff (a German physicist) discovered radium in 1861 while they were analyzing samples of the mineral lepidolite (KLi2Al(Al, Si)3O10(F, OH)2) with a device called a spectroscope.
- The sample revealed a set of deep red spectral lines that they had never seen previously. Bunsen was eventually able to extract rubidium from metal samples.
- Bunsen and Kirchhoff needed to process 150 kilograms (330 lb) of ore to extract enough rubidium to analyze its chemical properties since its content in the lepidolite was so low.
- In 1928, a sample of pure rubidium metal was created.
- Because of the vivid red lines in its emission spectra, they picked the term “rubidus”, which means “deep red” in Latin.
Occurrence of Rubidium
- Rubidium is the 23rd most prevalent element in the Earth’s crust, roughly equal to zinc but quite more common than copper.
- It is found naturally in minerals such as leucite, pollucite, carnallite, and zinnwaldite, which contain up to 1% rubidium oxide.
- The most common commercial resource for rubidium is lepidolite, which contains 0.3% to 3.5% of the element.
- Some potassium minerals and potassium chlorides also have commercially significant amounts of the element.
- Rubidium generally comes as a byproduct of the manufacturing of lithium.
Isotopes Of Rubidium
There are two naturally occurring stable isotopes of Rubidium which are: 85Rb and 87Rb
Naturally occurring rubidium isotopes are:
| Isotopes | Natural abundance (atom %) |
|---|---|
| 85Rb | 72.17 (2) |
| 87Rb | 27.83 (2) |
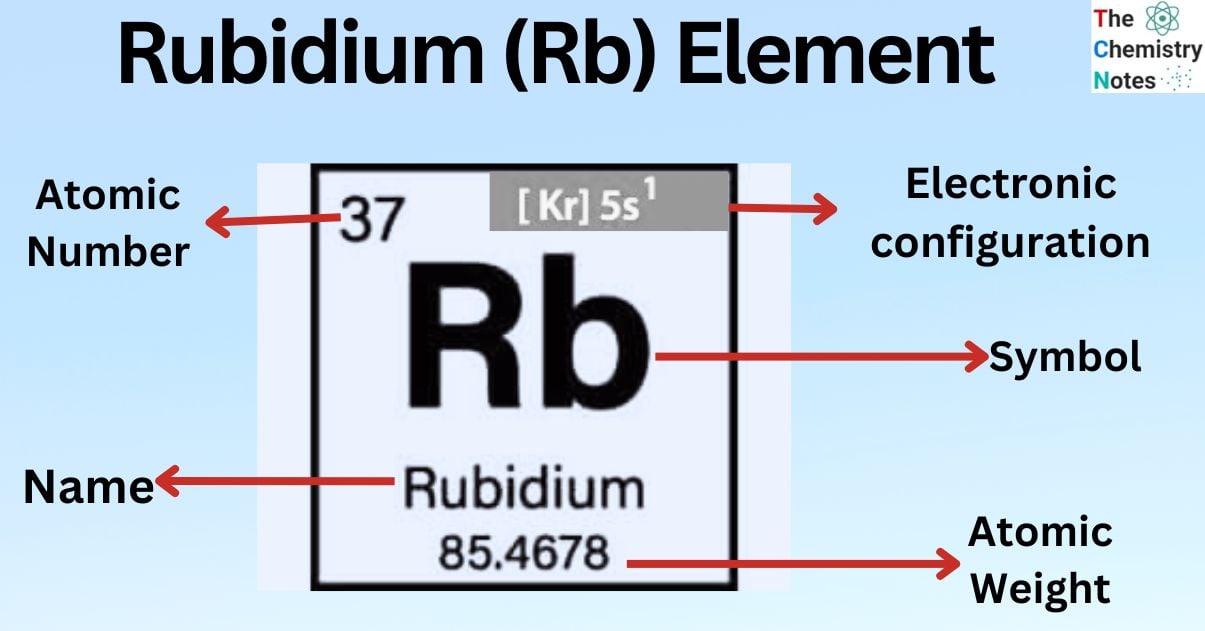
Elemental Properties of Rubidium
| Electronic Configuration | [Kr] 5s1 |
| Atomic Number | 37 |
| Atomic Weight | 85.468 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | 1, 5, s-block |
| Density | 1.53 g.cm -3 at 20 °C |
| Ionic radius | 0.149 nm (+1) |
| Van der Waals radius | 0.243 nm |
| Electron shells | 2,8,18,8,1 |
| Electrons | 37 |
| Protons | 37 |
| Neutrons in most abundant isotope | 48 |
Physical Properties of Rubidium
- Rubidium is a silvery-white, incredibly soft metal that is also one of the most reactive elements on the periodic table.
- It has a melting point of 39.30 °C (102.74 °F) and a boiling point of 688 °C (1270 °F).
- The density of Rubidium is 1.532 grams per cubic centimeter.
- Rubidium is the second most electropositive alkali metal.
- Rubidium serves as a good electrical conductor. Because electrons in rubidium are free to move around they are able to carry electrical charge from one end to other.
- Rubidium is an excellent thermal conductor as well. Heat causes a metal’s particles to vibrate more rapidly and move around more swiftly. Energy is transferred from one particle to another as they come into contact.
- Rubidium is ductile which means it can be drawn into thin wire without breaking it.
- It has no odor.
| Color/physical appearance | Silvery white |
| Melting point/freezing point | 39.30°C, 102.74°F, 312.45 K |
| Boiling point | 688°C, 1270°F, 961 K |
| Density | 1.53 g cm-3 at 20°C |
Chemical Properties of Rubidium
- Rubidium readily ignites in air and strongly reacts in water, igniting the liberated hydrogen.
- Rubidium reacts with the halides to create salts.
- It can form alloys with gold, sodium, potassium, and cesium.
Chemical Reaction Of Rubidium
- Reaction of Rubidium with Water
When rubidium metal combines with water, it forms a colorless solution of rubidium hydroxide RbOH and hydrogen gas H2. Because of the soluble hydroxide, the outcome of the reaction is basic. This process is extremely exothermic. The reaction is so quick that if it occurs in a glass vessel, the glass container may shatter. The reaction is slower than that of cesium but faster than that of potassium.
2Rb (s) + H2O (l) → 2RbOH (aq) + H2 (g)
- Reaction of Rubidium with Air
Rubidium is a soft metal that can be easily sliced. The finished surface is brilliant and lustrous. This surface, however, quickly tarnishes because it comes into contact with oxygen O2 and moisture in the air. When rubidium is burned in the air, it mostly produces dark brown rubidium superoxide, RbO2.
Rb (s) + O2 (g) → RbO2 (s)
- Reaction of Rubidium with the Halogens
Rubidium metal aggressively interacts with all halogens to generate rubidium halides.
Rubidium(I) fluoride, RbF, is formed when rubidium interacts with fluorine, F2.
2Rb (s) + F2 (g) → RbF (s)
Rubidium (I) chloride, RbCl, is formed when rubidium interacts with chlorine, Cl2.
2Rb (s) + Cl2 (g) → RbCl (s)
Rubidium (I) bromide, RbBr, is formed when rubidium interacts with Bromine, Br2.
2Rb (s) + Br2 (g) → RbBr (s)
Rubidium (I) iodide, RbI, is formed when rubidium interacts with Iodine,I2.
2Rb (s) + I2 (g) → RbI
- Reaction of Rubidium with Acid
Rubidium metal quickly dissolves in dilute sulphuric acid to generate solutions containing the aquated Rb(I) ion and hydrogen gas, H2.
2Rb (s) + H2SO4 (aq) → 2Rb+ (aq) + SO42- (aq) + H2 (g)
- Reaction of Rubidium with Bases
Rubidium does not react with bases under normal conditions.
Uses Of Rubidium
Rubidium and its compounds are used in different kinds of applications. some of which are mentioned below:
Used In Photoelectric Shells: Photoemissive surfaces containing rubidium and tellurium are employed in photoelectric cells that are employed in a range of electronic detection and activation devices. It is due to rubidium’s photoemission property, which is that of surface-emitting free electrons when struck by electromagnetic radiation.
Used In Atomic Clock: A new type of atomic clock is being developed using rubidium, which can be used as an alternative to cesium. Atomic clocks are the most precise time and frequency standards available, serving as a frequency standard for international time dissemination services, controlling the wave frequency of television transmissions, and being used in global navigation satellite systems such as GPS.
Used In Satellite Navigation and Communication: The Rubidium-87 atom’s frequency of resonance serves as a standard bandwidth in frequency standards and oscillators used in radio and television transmitters, telecommunication network synchronization, and satellite navigation and communication.
Used In Glasses: The largest market for rubidium is glasses, that are used in fiber optics telecommunications systems and night-vision devices. Rubidium Carbonate (Rb2CO3) is added to these types of glass to lower electrical conductivity and improve stability and durability.
Some other uses of rubidium are
Rubidium salts are used to add a purple hue to glassware, ceramics, and fireworks. Potential applications include ion engines for space vehicles, vapor turbine working fluid, and vacuum tube getters.
Rubidium is useful for monitoring ischemia, a condition in which blood flow through the main coronary arteries is restricted.
Health Effects Of Rubidium
- Normal human adults have roughly 300 mg in all tissues, which is more than most other ultra-trace elements. It also serves as a potassium replacement in the diet.
- Thermal burns may occur when rubidium ignites. Rubidium quickly combines with skin moisture to generate rubidium hydroxide, causing chemical burns to the eyes and skin.
- Because rubidium accumulates more in brain tumors than in normal brain tissue, nuclear medicine can use the radioisotope rubidium to identify and photograph tumors in the brain.
- Rubidium was also studied for its ability to treat manic depression. Rubidium has been detected in dialysis patients who suffer from depression, therefore supplementation may be beneficial during the depression.
Environmental Effects of Rubidium
There have been not any claims of harmful impacts on the environment.
Watch out for the video. Please note that this video was made solely for demonstration purposes! Do not attempt to repeat the experiments shown in this video!
References
- Lewis, G. M. (1952). “The natural radioactivity of rubidium”. Philosophical Magazine. Series 7. 43 (345): 1070–1074. doi:10.1080/14786441008520248.
- Campbell, N. R.; Wood, A. (1908). “The Radioactivity of Rubidium”. Proceedings of the Cambridge Philosophical Society.
- https://www.lenntech.com/periodic/elements/
- https://www.chemicool.com/elements/rubidium.html
- J W Mellor, A Comprehensive Treatise on Inorganic and Theoretical Chemistry., 1927, volume 2, Longmans, Green and Co., p422.
- https://www.rsc.org/periodic-table/element/37/rubidium
- Verhoeven, J.D. (1975) Fundamentals of Physical Metallurgy, Wiley, New York
- Greenwood, N. N.; Earnshaw, A. (1997). Chemistry of the Elements (2nd ed.). Oxford: Butterworth-Heinemann. ISBN 978-0-7506-3365-9.
- Hampel, Clifford A. (1968). The Encyclopedia of the Chemical Elements. Van Nostrand Reinhold. ISBN 978-0-442-15598-8.

