The term saponification incorporates the Latin term ‘sapo’, which translates to soap. Saponification is a broad term used to describe the chemical reaction involved in the production of soap. Soaps consist of extended molecular structures composed of Sodium (Na) and Potassium (K) salts of fatty acids.
Saponification is a chemical process in which triglycerides undergo an interaction with lye made from sodium or potassium hydroxide resulting in glycerol as well as a fatty acid salt commonly referred to as “soap.” Triglycerides commonly consist of animal fats or vegetable oils. The utilization of sodium hydroxide results in the formation of solid soap. The utilization of potassium hydroxide leads to the formation of soap with a delicate consistency.
Interesting Science Videos
History of Saponification
- The origins of soap manufacturing can be traced back to ancient times, spanning several millennia.
- Archaeologists have unearthed clay tablets in Mesopotamia dating back to 3000 BC that bear inscriptions detailing the process of soap making. Additionally, there exists compelling evidence of soap utilization within the early civilizations of both Rome and Egypt.
- During the 17th century in Europe, the emergence of Louis Pasteur’s discoveries led to a significant shift toward the promotion of proper hygiene practices.
How Saponification Works
- Saponification is classified as an exothermic chemical process characterized by the release of heat, which takes place upon the interaction between fats or oils (comprising fatty acids) and lye, an alkaline substance.
- During this chemical reaction, triglycerides, which are components present in fats, undergo a process of transformation when they react with either sodium hydroxide or potassium hydroxide. As a result, the triglycerides are transformed into soap and glycerol. In certain instances, the addition of salt is employed to induce the precipitation of solid soap.
- The process of saponification typically requires a duration of approximately 24 to 48 hours for completion, following the mixing of lye and oils and the subsequent pouring of the resulting raw soap into the designated mold. The rate of this process can be accelerated by increasing the temperature or decelerated by maintaining a lower temperature.
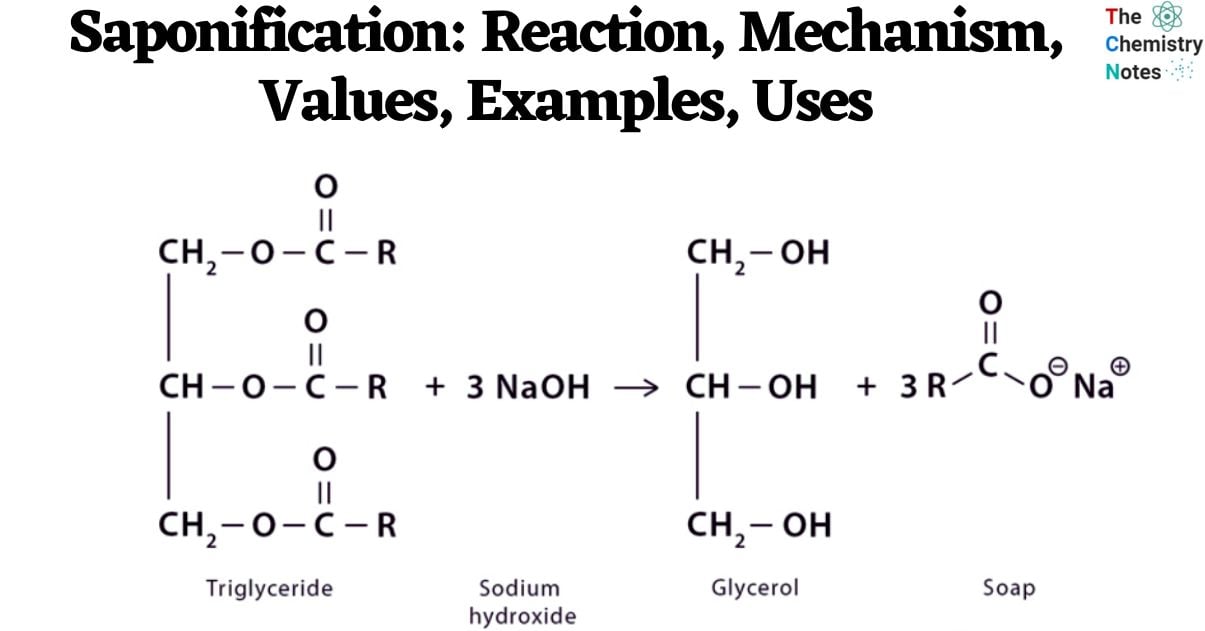
Saponification Reaction
The alkali employed in this procedure consists of sodium hydroxide (NaOH) or lye for the production of hard soap, and potassium hydroxide (KOH) for the creation of soft soap. Triglycerides typically encompass animal fats and vegetable oils. The reaction of sodium hydroxide with certain substances results in the formation of a solid variant of soap. When an alkali is introduced to water, esters undergo hydrolysis to yield alcohol and an alkali-metal salt of a Carboxylic acid.
The equation may be expressed as follows:
Ester + base → alcohol + soap
When employing Sodium Hydroxide (NaOH) as a reagent, the typical chemical reaction can be represented as follows:
RCOOR’ + NaOH → ROH + R’COO–Na+
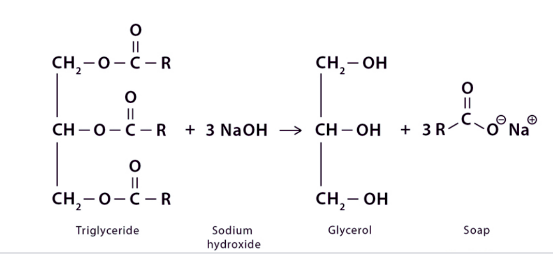
Saponification Mechanism
- The process of saponification requires a nucleophilic carbonyl substitution process, which can be elucidated through the following sequential steps:
- In the first step, the hydroxide ion, which is nucleophilic, attacks the ester group. This makes an intermediate compound.
- In the second step, the intermediate undergoes a cleavage reaction, resulting in the liberation of the leaving group. This process leads to the formation of a carboxylic acid and an alkoxide species.
- In the third step, the hydrogen atom in the carboxylic acid is removed using deprotonation. This makes a carboxylate ion and an alcohol.
Saponification Values
- The saponification value (SV) or saponification number (SN) is a number that shows how much potassium hydroxide (KOH) is needed to turn one gram of fat into soap under certain conditions.
- The saponification value is a crucial parameter utilized for the characterization and assessment of the quality of edible fats and oils.
- Additionally, the saponification number offers insights into the average molecular weight of the fatty acids.
- As the numerical value increases, the molecular weight of all fatty acids decreases.
- The saponification value is expressed in milligrams of potassium hydroxide per gram (mg KOH/g).
- If sodium hydroxide is employed in the saponification procedure, it is necessary to convert the saponification value from potassium to sodium. This can be achieved by dividing the values of potassium hydroxide (KOH) by the ratio of the molecular weights of KOH and sodium hydroxide (NaOH), which is approximately 1.403.
The following table displays the saponification values for different oils and fats:
| Oil/Fat | Saponification Value (mg KOH/g) |
|---|---|
| Palm Oil | 190 – 209 |
| Castor Oil | 176 – 187 |
| Olive Oil | 184 – 196 |
| Canola Oil | 182 – 193 |
| Soyabean Oil | 187 – 195 |
| Sunflower Oil | 189 – 195 |
| Palm Kernal Oil | 230 – 254 |
| Coconut Oil | 248 – 265 |
| Cotton Seed Oil | 189 – 207 |
Examples of Saponification
Sodium Stearate
- Sodium stearate, chemically represented as C18H35NaO2, is the sodium derivative of stearic acid, denoted as C18H36O2. This substance serves as both a soap and a detergent.
- It serves as a crucial ingredient in soap formulations, encompassing both a hydrophobic and a hydrophilic segment.
- The substance is formed via the hydrolysis of glyceryl tristearate, a triglyceride derived from stearic acid ((C18H35O2)3C3H5). This hydrolysis process involves the use of an aqueous solution of sodium hydroxide (NaOH).
(C18H35O2)3C3H5 + 3 NaOH → C3H5(OH)3 + 3 C18H35O2Na
Sodium Palmitate
- Sodium palmitate, chemically represented as C16H31NaO2, is classified as the sodium derivative of fatty palmitic acid (C16H32O2). This substance is commonly present in soaps and detergents.
- The derivation of the compound can be accomplished through the process of saponification, wherein glyceryl palmitate (C16H31O2)3C3H5) is reacted with sodium hydroxide (NaOH) in the form of caustic soda, lye, or lime.
(C16H31O2)3C3H5 + 3 NaOH → C3H5(OH)3 + 3 C16H31O2Na
Methyl Salicylate
- The chemical compound methyl salicylate (HOC6H4COOCH3) undergoes a reaction with sodium hydroxide (NaOH), leading to the formation of a dense white solid commonly referred to as sodium salicylate (HOC6H4COO–Na+).
HOC6H4COOCH3 + NaOH → HOC6H4COO–Na+ + CH3OH
Methyl Benzoate
- The chemical compound known as methyl benzoate (C8H8O2) undergoes a reaction when it comes into contact with an aqueous solution of sodium hydroxide (NaOH).
- This reaction results in the formation of sodium benzoate, a compound that is soluble in water, as well as methanol, a substance that is capable of mixing uniformly with water.
C8H8O2 + NaOH → C7H5O2Na + CH3OH
Methyl Acetate
- In the presence of sodium hydroxide (NaOH), the chemical compound methyl acetate (CH3COOCH3) undergoes saponification, resulting in the formation of sodium acetate (CH3COO−Na+).
CH3COOCH3 + NaOH → CH3COO−Na+ + CH3OH
Uses of Saponification
- One of the significant advantageous outcomes of saponification is observed in the context of fire extinguishers. The process of saponification is employed in wet chemical fire extinguishers to transform burning fats and oils into non-combustible soap, thereby aiding in the suppression of fires. Additionally, it is important to note that the reaction is classified as endothermic, meaning that it actively absorbs heat from the surrounding environment, resulting in a decrease in the temperature of the flames. This is due to the fact that cooking oils and fats possess a flashpoint that exceeds 37 degrees, thereby rendering conventional fire extinguishers ineffective.
- Saponification can be used for the production of hard and soft soaps. The modification of the reaction product between hard and soft materials can be accomplished by utilizing different types of alkali compounds throughout the process. Potassium hydroxide (KOH) can be utilized to obtain soft soaps. Sodium hydroxide can be used to produce solid soaps.
- Saponification holds great importance within the food industry as it enables the determination of the quantity of free fatty acids present in a given food product. The level of free fatty acid content can be discerned by quantifying the volume of alkali introduced to the fat or oil in order to achieve neutrality.
Video on Saponification
References
- https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/saponification#:~:text=Saponification%20is%20a%20process%20involving,the%20saponification%20value%20of%20fat.
- https://byjus.com/chemistry/saponification/
- https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Esters/Reactivity_of_Esters/Saponification
- Silvia A. Centeno; Dorothy Mahon (Summer 2009). Macro Leona, ed. “The Chemistry of Aging in Oil Paintings: Metal Soaps and Visual Changes.” The Metropolitan Museum of Art Bulletin. Metropolitan Museum of Art. 67 (1): 12–19.
- Helmenstine, Anne Marie, Ph.D. “Saponification Definition and Reaction.” ThoughtCo, Aug. 27, 2020, thoughtco.com/definition-of-saponification-605959.
- Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, ISBN 978-0-471-72091-1
- “Quality Laboratory Oil Examination Procedures and Practices”. American Oil Chemists’ Society. Archived from the original on 25 December 2012. Retrieved 17 December 2012.
- https://www.thesprucecrafts.com/saponification-in-soap-making-517092
- https://www.chemistrylearner.com/saponification.html