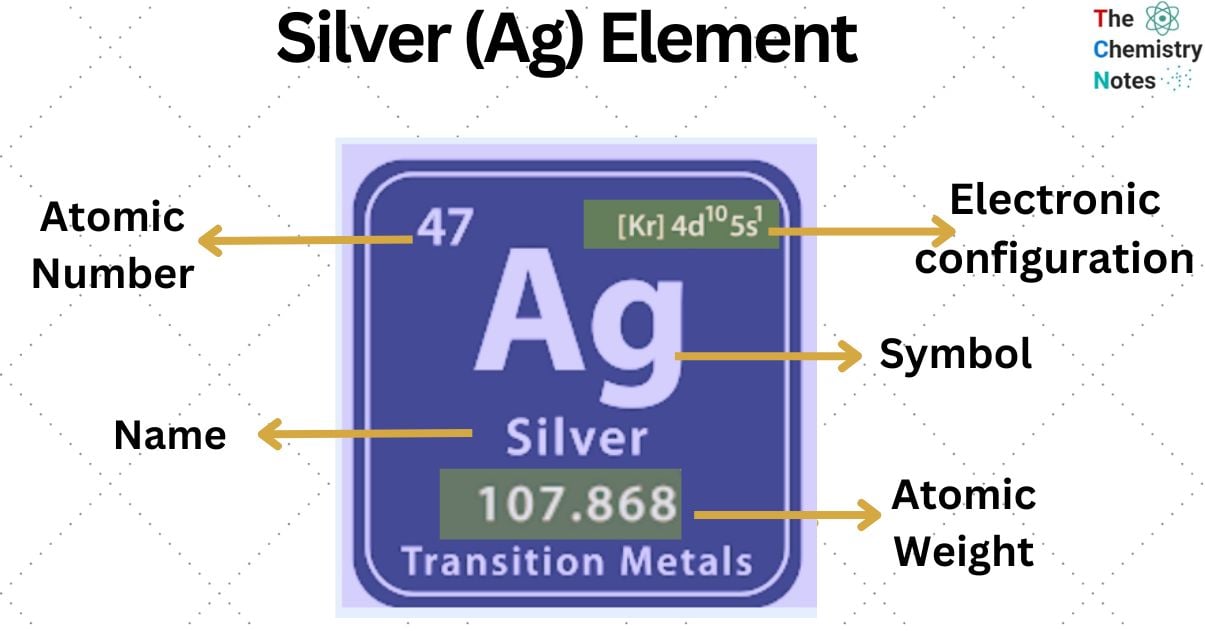
Silver is a metallic element with the atomic number 47 and is represented by the symbol ‘Ag’ in the periodic table. It is classified as a transition metal and belongs to the d-block of group 11 of the periodic table. It is soft and has brilliant metallic white luster.
Silver is a free element that is found in the Earth’s Crust. Its abundance is 0.08ppm (Part per million). Of all the known metals, silver is the one with the highest conductivity for electricity and heat. Silver occurs natively and in ores such as argentite (Ag2S) and horn silver (AgCl).
Interesting Science Videos
History Of Silver
- Silver has a long history that spans thousands of years. The precise discovery of silver has been lost to history, despite it being one of the seven ancient metals that date back to the prehistoric era.
- The evidence shows that silver were first mined in around 3000 BCE, in the modern day turkey and Greece.
- Since silver is more reactive than gold, supplies of native silver were much more limited than those of gold. Silver was more expensive than gold in Egypt until around 1500 BCE when the Egyptians are thought to have separated the gold from silver.
- Cupellation, a method for separating silver metal from its ores, was a game-changer since it revolutionized the scenario.
- Around 1,200 BCE, silver production relocated to the Laurium mines in Greece, where it continued to fuel the expanding empires in the area and even served as currency for ancient Athens.
- By 100 CE, Spain was home to the majority of the silver mines. These mines served as a major source of silver for the Roman Empire and a significant trading center for goods traveling over the Asian spice routes.
- The element gets its name from the Anglo-Saxon word ‘seolfor,” and its symbol ‘Ag’ derives from the Latin word ‘Argentum’.
Occurrence Of Silver
- The abundance of silver in the Earth’s crust is 0.08 ppm, almost the same as that of mercury.
- It typically appears in nature in the form of sulfides, such as galena (lead sulfide) or cerussite (lead carbonate), as well as in minerals containing silver compounds.
- Argentite (Ag2S), chlorargyrite (Agcl), and pyrargyrite (Ag3SbS3) are the three main silver ores.
Argentite (Ag2S): Argentite is one of the most important silver ore minerals after galena. It is the sulfide ore of silver, which is otherwise known as silver glance because of its high silver content.
Chlorargyrite (Agcl): Silver chloride (AgCl) is present in it as a mineral. Chlorargyrite, which is usually found in dry regions, occurs in oxidized areas above silver deposits and can be a valuable resource in some occasions.
Pyrargyrite (Ag3SbS3): Pyrargyrite is one of the rare silver-bearing minerals that can display transparency. Silver sulfantimonite, Ag3SbS3, makes up this sulfosalt mineral. It is also referred to as ruby silver.
The major commercial sources of silver are the ores of copper-nickel, lead, copper, and lead-zinc, which are obtained from Australia, Mexico, Bolivia, Peru, Poland, and Chile, among others.
Isotopes Of Silver
Silver consist of two naturally occurring stable isotopes: 107Ag and 109Ag.
Natural isotopes of Silver
| Isotope | Natural abundance (atom %) |
|---|---|
| 107Ag | 51.839 (7) |
| 109Ag | 48.161 (7) |
Elemental Properties of Silver
| Electronic Configuration | [Kr] 4d10 5s1 |
| Atomic Number | 47 |
| Atomic Weight | 107.868 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | 11, 5, d-block |
| Density | 10.49 g.cm -3 at 20 °C |
| Ionic radius | 0.126 nm |
| Van der Waals radius | 0.144 nm |
| Electron shells | 2, 8, 18, 18, 1 |
| Electrons | 47 |
| Protons | 47 |
| Neutrons in most abundant isotope | 61 |
Physical Properties of Silver
- Silver has an atomic number of 47 and is a silvery-white metal. It has a melting point of 961.78 °C (1763.2 °F) and a boiling point of 2162 °C (3924 °F).
- Silver has a solid phase density of 10.49 gm/cm3 and a liquid or molten phase density of 9.320 gm/cm3.
- Silver is non-magnetic metal.
- Silver is an extremely malleable metal, which allows it to be easily hit into sheets without cleavage.
- Silver is also a ductile metal, which makes it possible to draw thin wires without breaking them. It is only second to gold in terms of ductility.
- Silver serves as an excellent electrical conductor. Its electrical conductivity is the greatest of all metals, even greater than that of copper. Because electrons in iron are free to move around they are able to carry electrical charge from one end to other.
- Silver is an extremely good thermal conductor, which is the highest among all other metals. Heat causes a metal’s particles to vibrate more rapidly and move around more swiftly. Energy is transferred from one particle to another as they come into contact.
- Since silver does not rust under normal circumstances, it is also a metal that resists corrosion.
| Color/physical appearance | Silvery-White |
| Melting point/freezing point | 1234.93 K (961.78°C, 1763.2°F) |
| Boiling point | 2435 K (2162°C, 3924°F) |
| Density | 10.5 g cm-3 at 20° |
| Malleability | Yes |
| Ductility | Yes |
| Electronegativity | 1.93 (Pauling Scale) |
Chemical Properties of Silver
- Even when heated until it becomes red, silver does not react with air.
- The first ionization energy of silver is the lowest compared to all other elements in Group 11.
- The reactivity of silver is between copper and gold.
- Silver forms a halide with all four halogens, and only with fluorine does it form a dihalide.
- The most common oxidation states of silver are +1 and +2. The state of +3 is also rarely observed.
- It rarely reacts because it lacks chemical activity, but nitric and hot concentrated sulfuric acid is capable of attacking it.
Chemical Reaction of Silver
- The reaction of Silver with Air
Silver does not react with clean air under normal condition.
- The Reaction of Silver with water
Silver does not react with water.
- The Reaction of Silver with the Halogens
When a silver metal reacts with fluorine, F2, a thermally stable difluoride, silver (II) difluoride, AgF2, is formed.
Ag (s) + F2 (g) → AgF2 (s) [brown]
Uses of Silver
When compared to the other precious metals silver is cheaper and more readily available. Silver can be made into paste, powder, flakes, salt, stretched into wires, flattened into printable sheets, suspended as a colloid, or even used as a catalyst. It can also be alloyed with different metals. Therefore silver has various applications. Some of these are included here:
Used In Electronics Industry: The primary application of the silver is in the electronics industry. Because of its superior thermal and electrical conductivity compared to other metals, silver cannot be simply replaced with less expensive alternatives. It is used as a contact in electrical switches. Smartphones, which have become an integral part of our lives, have LED chips and touch-sensitive displays that use silver.
Used In the Automobile Industry: Electrical connections used in a modern car are activated with the help of silver-coated contacts. Silver membrane switches are often used to ignite the engine, open and close the power windows, open and close the trunk, and adjust the seats. It is also frequently used in high-end spark plugs and antifreeze for vehicles.
Used In Engine Bearings:Steel utilized in the production of bearings is electroplated with silver. It has the property of a high melting point. These bearings are strong enough to withstand the intense heat from the engine. Additionally, silver can function as a lubricant, lowering friction between a ball bearing and its housing.
Used In Solar Panels: Silver possesses the extraordinary property of being ground into a paste. This silver paste is used in solar panels. It is generally contacted with printed photovoltaic cells, which can attract and carry the electric current. Silver is a reflective metal that allows it to reflect the energy into collectors, which then use salts to generate electricity.
Used In Batteries: Silver oxide and silver-zinc alloys are used in the batteries for better performance at high temperatures. Power cameras, aerospace equipment, defense equipment, and watches use silver oxide batteries. Silver zinc is also used for laptop batteries, and electric cars in place of lithium batteries.
Used In Brazing And Soldering:Due to its high tensile strength and ductile properties, silver is often used for brazing and soldering. It can help to create a tight joint between air conditioning vents and plumbing. Antibacterial properties and non-toxicity to humans make silver an excellent replacement for lead-based bonds in water pipes. Brazing is done at temperatures above 600 °C, whereas soldering is executed below 600 °C.
Used In Jewelry: Silver is perfect for manufacturing jewelry and rings since it is valuable, attractive, and durable. It was and continues to be utilized to produce jewelry. Silver is more affordable than gold, which makes it a suitable material for jewelry. Rings, dishes, and silverware are some examples of silver jewelry. Silver rings are often chosen for their timeless qualities, gloss, and consistency.
Used In Mirrors And Glasses: Silver can reflect light when it is polished. Most modern buildings nowadays use silver-coated windows to reflect sunlight and UV rays. The glass layer used in mirrors is coated with a thin silver layer to provide the desired reflectivity. Usually, people living in hot regions use these glasses to maintain their temperature. It can also be cost-effective, as it can reduce the energy bills of the air conditioner.
Used In Medicines: Silver ions can act as catalysts by absorbing oxygen and killing bacteria by interfering with respiration. Silver foil was wrapped around wounds to help them heal before the antibiotics came into use. Colloidal silver and silver-protein complexes were ingested or applied topically to fight illness. Silver is also used in eye drops and dental hygiene to cure and prevent infections.
Health Effects Of Silver
- Photographers use powder with silver, which may come into contact with the skin. Repeated exposures to silver compounds can cause skin and other body tissues to turn gray or blue-gray. This condition is called argyria, which is harmless but permanent cosmetically.
- Eye contact can cause severe corneal injuries when liquid comes into contact with the eyes.
- Skin contact can cause skin irritation. Repeated and prolonged contact with the skin can cause allergic dermatitis.
- Exposure to high concentrations of vapors can cause dizziness, breathing difficulty, headaches, or respiratory irritation. Extremely high concentrations can cause drowsiness, staggering, confusion, unconsciousness, coma, or death.
- Aspiration of material into the lungs, when swallowed or vomited, it can cause chemical pneumonitis, which can be fatal.
Environmental Effects Of Silver
Silver is not regarded as an environmental risk in either its pure metal state or in ores because it does not disintegrate. However, it has been discovered that excessive quantities of some silver compounds are extremely harmful to aquatic life forms, such as fish.
- Silver use in many aspects of life and industry increases environmental pollution, including soil pollution. A lot less research has been done on the environmental effects of soil contamination from silver than has been done on the effects of other heavy metals.
- When removing microorganisms from sewage, silver interrupts the process.
- Silver stops sludge from being used as fertilizer, which is necessary for nutrient recycling.
- Since these forms of metal tend to react more readily with biological molecules, scientists once thought that metals that existed as free ions were more likely to be dangerous to living organisms.
- Studies on fish and zooplankton exposed to high concentrations of silver nitrate—a form of the metal that contains a lot of free ions—confirmed that silver is indeed extremely harmful to aquatic life. The potassium and sodium levels in fish are controlled by an enzyme called sodium/potassium ATPase, which is interfered with by this ionic form of silver.
- Fish swiftly lose ions from their circulation, water penetrates their bodily tissues, and they succumb to cardiovascular failure when the sodium/potassium equilibrium is disturbed.
Videos on Uses of Silver
References
- https://www.rsc.org/periodic-table/element/47/silver
- W. M. Haynes, ed., CRC Handbook of Chemistry and Physics, CRC Press/Taylor and Francis, Boca Raton, FL, 95th Edition, Internet Version 2015, accessed December 2014.
- John Emsley, Nature’s Building Blocks: An A-Z Guide to the Elements, Oxford University Press, New York, 2nd Edition, 2011.
- https://sites.dartmouth.edu/toxmetal/more-metals/silver-metal-of-many-faces/the-facts-on-silver/
- https://www.lenntech.com/periodic/elements/ag.htm
- Silver Ecotoxicity Estimation by the Soil State Biological Indicators. https://doi.org/10.1155/2020/1207210
- Silver Emissions and their Environmental Impacts: A Multilevel Assessment. https://doi.org/10.1021/es062970d

