Derived from the Latin name “natrium,” sodium is represented by the symbol Na and has the atomic number 11. Because sodium ions (Na+) are soluble in water, the seas contain huge amounts of these ions. Na+ is a component of minerals and a necessary component of all living things.
Sodium ions serve essential biological functions, primarily in maintaining human body fluids and facilitating neuronal activity for the transmission of nerve impulses. Na+ functions as a critical electrolyte and a vital component of extracellular fluid, actively participating in osmoregulation, a passive transport process. Additionally, Na+ plays a pivotal role in muscle contraction and the enzymatic activities of various enzymes. Within the human body, Na+ is frequently involved in actively creating an electrostatic potential across membranes, typically alongside potassium ions (K+) as counter-ions. The establishment of this electrostatic potential across cell membranes is crucial for enabling the transmission of nerve impulses.
Interesting Science Videos
Biological Significance of Sodium
Osmosis
Osmosis is the physical process by which a solvent (water) diffuses into a region of high solute (salt) concentration through a semi-permeable membrane. To dilute and equalize the concentrations, the solvent (water) moves over the semi-permeable membrane from one solution having a lower salt concentration towards a second solution with a higher salt concentration. This is known as the osmotic gradient.
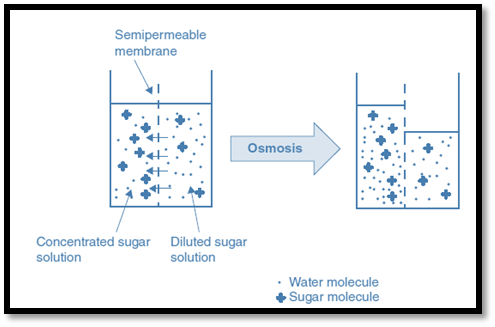
Osmosis
- Sodium holds a vital role as a necessary mineral in the human body, playing a crucial part in regulating body fluid through its osmotic activity.
- Sodium ions constitute over 90% of all ions in both plasma and interstitial fluid, actively participating in osmosis processes. As the predominant cation in extracellular fluid, Na+ content governs the extracellular volume, with the kidneys playing a pivotal role in regulating overall fluid levels.
- Specifically, the nephrons, the functional units of the kidney, are involved in the filtration, secretion, and re-absorption of Na+. The utilization of Na+ ions in the establishment of osmotic gradients is instrumental in controlling water balance within the human body.
- Additionally, the kidneys detect decreases in blood pressure and Na+ concentrations, triggering the release of hormones such as renin, antidiuretic hormones (ADHs), and atrial natriuretic peptide. These hormones play a key role in regulating blood pressure, osmotic balances, and mechanisms for retaining water.
- In general, if a medium is
- When a solution is hypertonic, it indicates that its solute concentration is higher than that of its surroundings. Osmosis will cause this area to lose water.
- If a solution is isotonic, it contains the same solute concentration as its surroundings. No water movement will take place.
- Hypotonic refers to a solution in which the concentration of solutes is lower than that of its surroundings. Through osmosis, water will be added to this space.
To minimize the concentration difference, there is a net migration of the solvent (water) from the hypotonic solution to the solution with the greater concentration. The pressure needed to create equilibrium without any solvent movement is known as the osmotic pressure. It is crucial to remember that osmotic pressure is determined by the quantity of ions or molecules present in the solution, not by their specific nature. The osmole (also known as osmol or osm), a non-SI unit that indicates the number of moles of a substance that contributes to the osmotic pressure of a solution, is frequently used to characterize the osmotic pressure.
Osmotic processes are generally critical to a variety of biological functions.
- Plants use the osmotic gradient to determine the turgor inside their cells and osmosis to move water and other solutes through their systems.
- Urine excretion is one of the most common processes in the human body that involves osmosis.
Generating urine happens inside the kidney, specifically at the kidney’s functional components, the nephrons. Urine is produced by filtering 150–180 mL of plasma through the glomerulus, a component of the nephron, each day. The distal tubule, which connects to the collecting duct and eventually the ureter, the Loop of Henle, and the proximal tubule make up the nephron.
Filtration occurs at the glomerulus, while the remaining segments of the nephron are responsible for regulating imbalances through the secretion and re-absorption of ions, managing urine volume before storage in the bladder. This process involves active or passive transport across the nephron membrane. Na+ is typically actively transported through Na+ pumps to establish the correct Na+ concentration in blood plasma, crucial for maintaining appropriate osmotic pressure. This active transport creates an osmotic gradient in the kidney parenchyma, aiding in water conservation.
The ascending limb of the Loop of Henle is impermeable to water but allows the passage of Na+, establishing an osmotic gradient. Conversely, the descending limb is permeable to water, allowing it to move to the interstitial fluid due to the osmotic gradient, resulting in concentrated urine. The collecting ducts become permeable to water under signals indicating the need for water conservation, and water, following the osmotic gradient, further concentrates the urine.
Active transport of sodium ions
The operation of neurons and the subsequent transmission of a nerve impulse depend on the active transport of sodium ions. Na+/K+ pumps can be used as the active unit to actively build up a concentration gradient throughout the cell membrane to accomplish this. The cells’ comparatively high potassium ion concentrations and low sodium ion concentrations are caused by this active transport. Nerve impulses are transmitted by the action potential, which is the electrostatic potential that develops along the cell membrane as a result.
- The breakdown of ATP drives conformational changes in the cross-membrane protein, which in turn drives an active transport mechanism facilitated by the Na+/K+ pumps.
- Three Na+ ions bind the cytosolic side of the cross-membrane protein in the first step.
- As a result, the protein modifies its structure and becomes ATP-accessible.
- A second conformational shift occurs when ATP phosphorylates the protein in its new confirmation.
- The protein now has a low affinity for the sodium ions, and the three Na+ ions are positioned across the membrane.
This indicates that the sodium ions are discharged into the extracellular fluid after becoming separated from the protein. However, the protein now binds two potassium ions from the extracellular fluid and has a high affinity for K+. Now that the bond phosphate has separated, the protein returns to its initial confirmation. This indicates that both K+ ions are free to be released and are exposed to the cytosol.
Drugs, Diet and Toxicity
When a patient is diagnosed with dehydration and sodium depletion, sodium chloride solutions are typically used.
- Most patients receive treatment intravenously, although oral sodium bicarbonate or chloride may be used in chronic circumstances (mild to moderate sodium loss).
- Alkali metal-based salts like NaCl, KCl, and related citrates are typically used in oral rehydration treatments.
Sodium bicarbonate is typically used orally to control the pH of the serum.
- PlasmapH imbalances may result from renal tubular acidosis or other kidney-related issues.
- This is a medical disorder where the kidneys fail to control the pH of the blood plasma and urine, causing the body to collect acid.
- Blood is filtered in the kidneys before it enters the tubular section of the nephrons, which is where vital salts and other substances are secreted or reabsorbed.
- Renal tubular acidosis occurs when the kidneys are unable to recover bicarbonate ions (HCO3–) from the filtrate (passive reabsorption occurs in the proximal tubule, active reabsorption occurs at the distal tubule), which is required to maintain pH balance, or filter or secrete acid ions (H+) from the plasma (secretion occurs in the distal tubule).
- According to this mode of action, the bicarbonate anion in sodium bicarbonate is the pharmaceutically active component, although the cation Na+ is in charge of solubility and compatibility.
Table salt is the most widely consumed dietary source of NaCl and is used for pickling and seasoning (the osmotic gradient caused by the high NaCl concentration prevents the growth of bacteria and fungi).
- Depending on the nation and age category, different daily intake recommendations for NaCl are made. In the UK, an adult’s maximum salt intake is advised to be no more than 6 g of NaCl, while children’s intake should be substantially lower. The majority of people regularly surpass this limit, and elevated salt plasma levels (hypernatraemia) can lead to heart conditions including hypertension.
- Hyponatraemia, or low sodium plasma levels, can also be caused by renal failure or salt loss in the colon. These conditions can equally harm the body by creating osmotic imbalances, and they may need to be addressed. Symptoms of a salt shortage include low blood pressure, cramping in the muscles, and dehydration. Acute poisoning symptoms could appear at 500–1000 mg/kg of body weight after consuming NaCl. These symptoms may include kidney damage, vomiting, and gastrointestinal (GI) tract ulcers. Moreover, a high salt diet is thought to raise the likelihood of kidney stones forming.
References
- Pasquale Strazzullo, Sodium, Advances in Nutrition, Volume 5, Issue 2, March 2014, Pages 188-190
- James L. Lewis III, Hypernatremia (High Level of Sodium in the Blood), MD, Brookwood Baptist Health and Saint Vincent’s Ascension Health, Birmingham.
- https://www.pharmacy180.com/article/sodium–an-essential-ion-in-the-human-body-1380/
