When two liquids can form a two-phase liquid system over a range of compositions, we say that they are partially miscible liquids. This is a typical case involving two liquids, one of which is polar and the other of which is non-polar (for example, water and vegetable oil). The mixture of diethyl ether and water is another common example in the field of organic chemistry. Using a separatory funnel, the two-phase liquid system can be utilized to separate solutes with varying solubility in the immiscible liquids.
The solubility of most solutes is dependent upon temperature. The miscibility of numerous binary mixtures comprising immiscible liquids tends to increase as the temperature increases. Subsequently, when reaching a specific temperature referred to as the upper critical temperature, the liquids achieve complete miscibility across all compositions.
Interesting Science Videos
Partially miscible liquids
Pressure, temperature, and composition are the most crucial factors in characterizing a mixture. Because composition is irrelevant in these cases, phase diagrams for single-component systems often simply show the effects of changes in pressure and temperature. However, in the case of two-component mixtures, where the composition is crucial, the other variable to be portrayed is typically either temperature or pressure. Many binary systems have two-phase compositions at different temperatures, and these compositions can be described using temperature-composition diagrams.
There exists a considerable quantity of liquids that exhibit limited solubility when trying to dissolve in one another, such as the case of ether and water. Ether exhibits a solubility of approximately 1.2% in water, while water demonstrates a solubility of approximately 6.5% in ether. Due to the restricted extent of their solubilities, these substances exhibit partial miscibility. When equal volumes of ether and water are agitated, two distinct layers are observed. One layer consists of a saturated solution of ether in water, while the other layer consists of a saturated solution of water in ether. The aforementioned pair of solutions is commonly known as conjugate solutions. The impact of temperature on the mutual solubility of these mixtures comprising conjugate solutions is of particular significance.
Types of partially miscible liquids
There are four types of partially miscible liquids:
- Partially miscible liquids in which an increase in temperature results in an increase in the partial miscibility of the substance. For example, systems including phenol and water, aniline and water, aniline and hexane, CH3OH and CS2, and cyclohexane and methanol. The liquids are able to combine perfectly with one another above a particular temperature threshold.
- Partially miscible liquids in which partial miscibility increases when the temperature is lowered. System compositions such as (C2H5)NH-H2O and (C2H5)3N-H2O are two examples. Two liquids are able to mix entirely with one another when the temperature is below a specific threshold.
- Partially miscible liquids in which the partial miscibility increases in particular temperature ranges both when the temperature is raised and when the temperature is lowered. H2O-nicotine and H2O-picoline are both examples of system types. Above and below a particular temperature, these two types of liquid are completely interchangeable with one another.
- Partially miscible liquids in which the temperature required for complete miscibility cannot be reached. For example, ether and water
The impact of temperature on the composition of three representative mixtures, focusing on the observed changes in their chemical compositions are described below:
- Phenol-Water system
- Triethylamine-Water system
- Nicotine-Water system
Phenol-water system
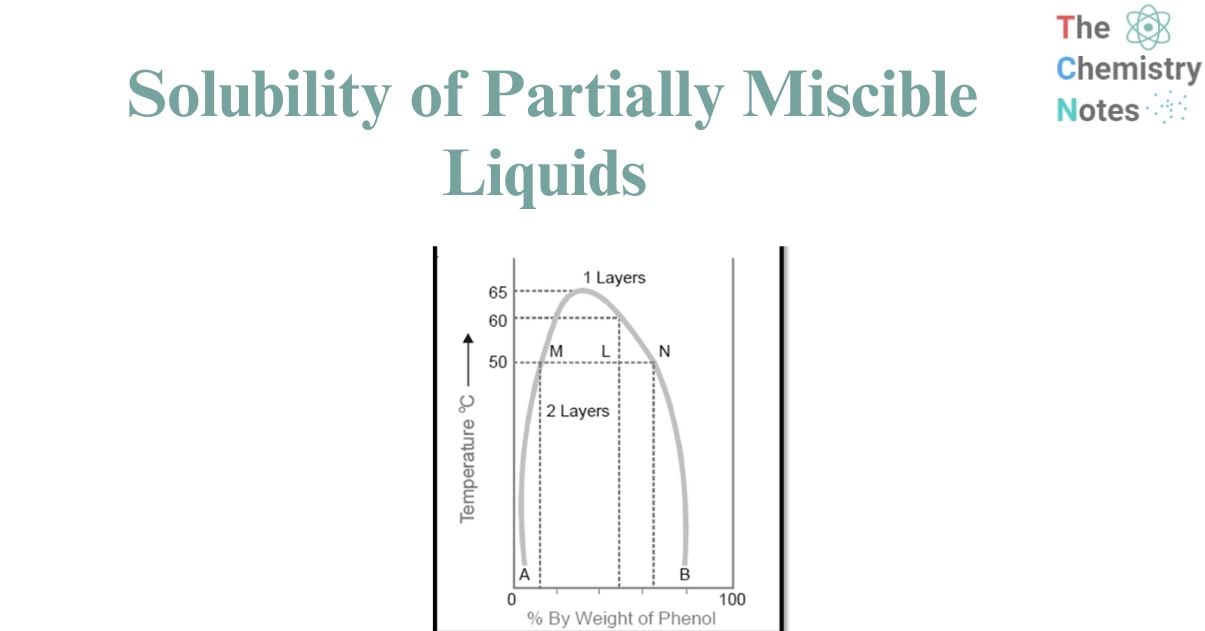
The diagram provided below illustrates the miscibility characteristics between phenol and water. The left side of the parabolic curve represents one of the two conjugate solutions, illustrating the varying percentage of phenol that is dissolved in water at different temperatures. The solubility of phenol exhibits a positive correlation with temperature.
- The right side of the curve corresponds to the additional conjugate solution layer, which provides the percentage of water present in phenol.
- The solubility of water in phenol also increases with an increase in temperature.
- The intersection of the two solution curves occurs at the points of maximum temperature on the temperature-composition curve of the system. The aforementioned point corresponds to a temperature of 66°C and a phenol composition of 33%.
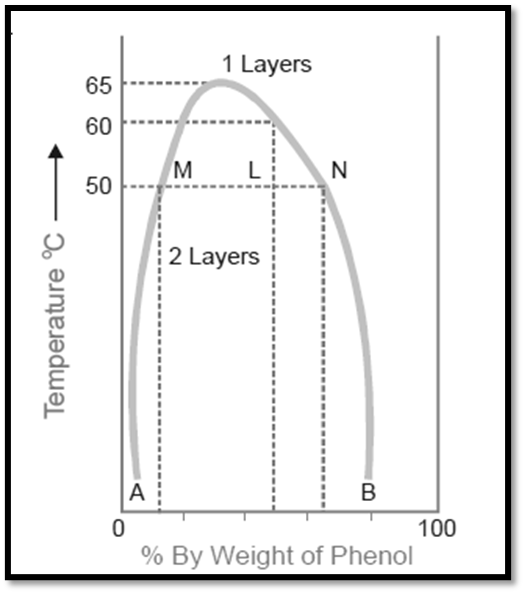
Figure: Phenol-water system
Therefore, when reaching a specific upper limit of temperature, the two conjugate solutions combine, resulting in their identical nature and the formation of a single layer. The Critical Solution Temperature (CST), also known as the Upper Consolate Temperature, refers to the temperature at which the two conjugate solutions or layers combine to form a single layer. This phenomenon exhibits particular characteristics specific to a given system and is significantly affected by the existence of impurities. The determination of the critical solution temperature can be employed to assess the purity of phenol and similar substances.
Phenol and water exhibit complete miscibility in all proportions when the temperature exceeds the critical solution temperature. Beyond the curve, the system exhibits a state of complete uniformity, wherein only a single layer is present. Conversely, below the curve, the degree of miscibility is contingent upon the specific composition of the mixture. Based on the Figure , it can be observed that when the temperature is below 50°C, a mixture consisting of 90% phenol and 10% water, or alternatively, 5% phenol and 95% water, will exhibit complete miscibility. This conclusion is drawn from the fact that the corresponding data points do not fall below the curve. The presence of two distinct layers can be consistently observed below the curve, with the curve itself indicating the compositions of the conjugate solutions that make up these two layers. At a temperature of 50°C, a mixture consisting of equal proportions of phenol and water (50% each) will undergo phase separation, resulting in the formation of two distinct layers with compositions referred to as A and B. The line connecting the points (M and N) that represent the compositions A and B is commonly referred to as the tie line. This equation facilitates the determination of the proportional quantities of the two layers, denoted by the ratio MN/ML in this context.
Trimethylamine–water system
The figure illustrates the temperature-composition curve depicting the mutual solubilities of triethylamine and water.
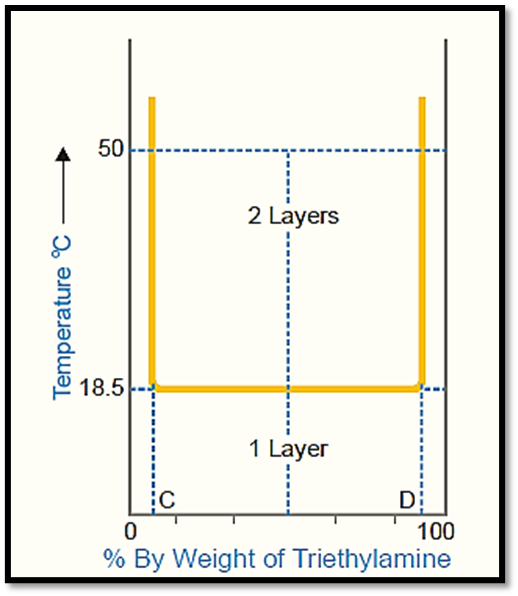
- The left side of the curve represents the solubility curve of triethylamine in water, while the right side represents the solubility curve of water in triethylamine.
- In contrast to the phenol-water system, the solubilities in this particular system exhibit a decrease as the temperature increases.
- The two conjugate solutions exhibit complete mixing when their temperature is at or below 18.5°C.
- The temperature here is alternatively referred to as the critical solution temperature or the lower consolidate temperature.
In the aforementioned scenario, it can be observed that any point situated above the horizontal line signifies the presence of heterogeneity within the system, characterized by the existence of two distinct layers. Conversely, points located below the line indicate complete homogeneity, denoting the presence of a single layer. Therefore, a mixture with equal proportions of components (50-50) will exhibit complete miscibility at a temperature of 10°C. However, at a temperature of 50°C, the mixture will undergo phase separation, resulting in the formation of two distinct layers with compositions corresponding to points C and D.
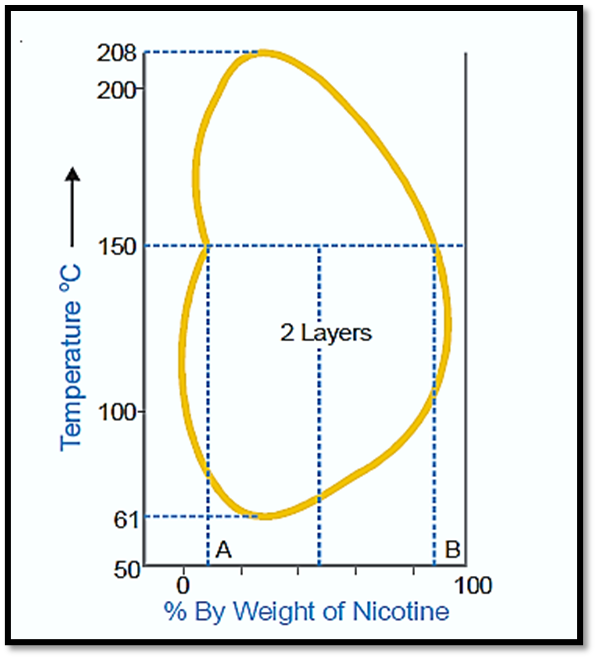
Nicotine-water system
The behavior exhibited by this particular system can be described as a fusion of characteristics observed in both the initial and secondary types. At ambient temperature, nicotine and water exhibit complete miscibility. However, at elevated temperatures, the mutual solubility between the two substances decreases. Upon further increase in temperature, the two liquids regain their miscibility. In essence, mutual solubility exhibits an increase in certain temperature ranges, regardless of whether the temperature is lowered or raised. Therefore, a closed solubility curve is observed in the system, indicating the presence of two critical solution temperatures: an upper temperature of 208°C and a lower temperature of 61°C. The impact of pressure on this particular system results in an increase in the lower critical temperature and a gradual decrease in the upper critical temperature, ultimately leading to their convergence. Currently, the liquids exhibit complete miscibility across all temperature ranges.
What is the critical solution temperature?
The temperature at which a pair of liquids that are only partially miscible becomes miscible in all proportions is referred to as the critical solution temperature (CST) or the consolute temperature for the pair. This temperature can be above (or below) room temperature.
- Upper critical solution temperature: After reaching a specific temperature, the solutions become capable of being miscible. The term “upper critical solution temperature” refers to this particular temperature.
- Lower critical solution temperature: Below a particular temperature, also known as the lower critical solution temperature, the total miscibility of the two substances will be obtained.
References
- https://www.sfscientific.com/science/2016/03/15/solubility-diagram-of-two-partially-miscible-liquids/
- https://www.tutorsglobe.com/homework-help/chemistry/partially-miscible-liquids-76679.aspx
- https://solutionpharmacy.in/partially-miscible-liquids/
- https://globalresearchonline.net/journalcontents/v54-1/19.pdf
- https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Physical_Chemistry_(Fleming)/08%3A_Phase_Equilibrium/8.06%3A_Phase_Diagrams_for_Binary_Mixtures
