Specific heat capacity is the amount of heat energy required to raise the temperature of a substance per unit of mass. A material’s specific heat capacity is a physical characteristic. It is also an example of an extensive property because its value is proportional to the size of the system under consideration.
Interesting Science Videos
What is Heat Capacity?
The amount of heat energy required to raise the temperature of a given quantity of matter by one degree Celsius is known as its heat capacity. Heat capacity is a broad characteristic of matter since it changes with size and quantity.
The heat capacity of a system is a measure of its entire internal energy. This includes both the overall kinetic energy of the system and the potential energy of the molecules. It has been proved that the internal energy of a system can be changed by either applying heat energy to it or performing work on it. As the temperature rises, the internal energy of a system increases. Temperature differences and the amount of internal energy influence this increase.
The heat capacity can be calculated mathematically as follows:
c = Q /ΔT
where,
- Q is the amount of heat energy required to change the temperature (ΔT).
- c is the heat capacity of the system
What is Specific Heat Capacity?
Specific Heat Capacity is one of the fundamental properties of matter that describes the amount of heat energy essential to raise the temperature of 1 kilogram of matter by 1 degree Celsius. The SI unit for Specific Heat Capacity is J/(KgK), however Specific Heat Capacity is also measured in cal/(gK) or J/(g°C). The specific heat capacity of any matter depends on its composition, density, and physical state, just as the specific heat of water varies.
- The specific heat capacity of each liquid varies; for example, raising the temperature of water by 1°C requires a different quantity of heat energy than raising the temperature of another liquid.
- If a substance requires an immense quantity of energy to heat up, its specific heat capacity will be very high. These substances will be able to store a significant amount of energy.
- If a substance requires a small quantity of energy to heat up, its specific heat capacity will be quite low. These substances will not be able to store a large amount of energy.
Formula of Specific Heat Capacity
Q = C m ∆t
Where,
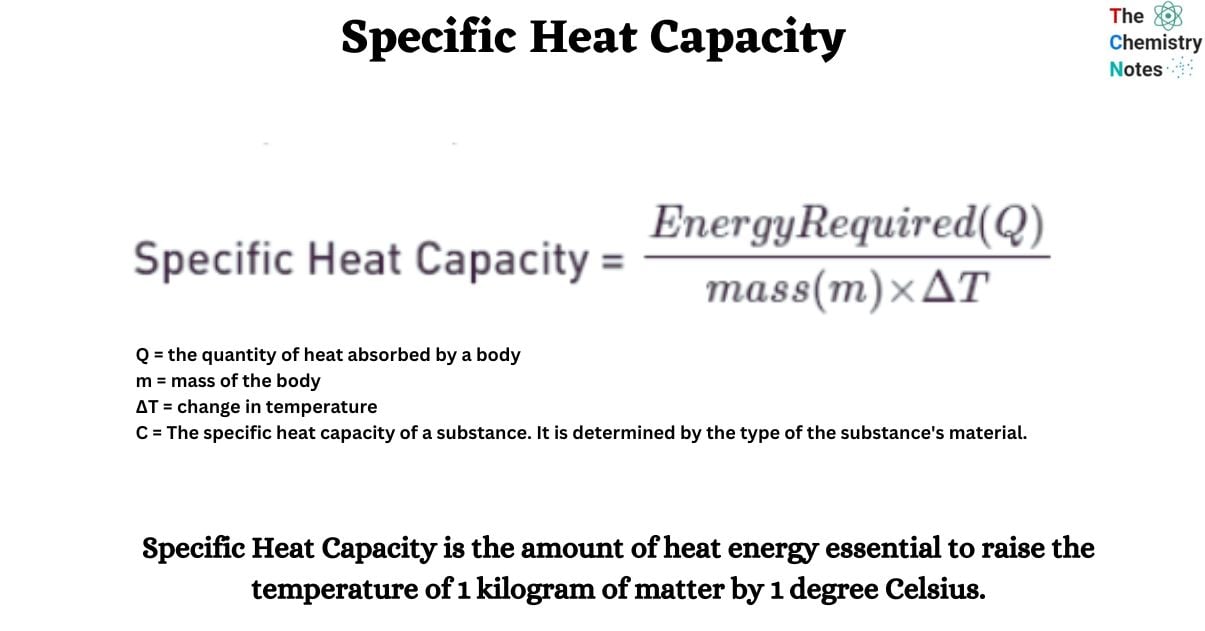
- Q = the quantity of heat absorbed by a body
- m = mass of the body
- ∆t = change in temperature
- C = The specific heat capacity of a substance. It is determined by the type of the substance’s material.
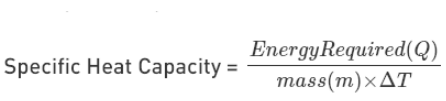
Unit of Specific Heat Capacity
The quantity of heat required to raise the temperature per unit mass is referred to as specific heat capacity.
The S.I unit for Specific Heat Capacity is JKg-1K-1, whereas, the SI unit for heat capacity is J.K-1.
Specific Heat Capacity of Water
Some compounds heat up quickly, while others heat up slowly. Water is one of the substances with a high specific heat capacity since it takes more energy to raise the temperature. for example, has a specific heat of 449 J/kg°C, sand has a specific heat of 830 J/kg°C, and oak lumber has a specific heat of 2400 J/kg°C.
The specific heat capacity of water is 4182 J/kg°C. Due to the significance and prevalence of water, we even have a particular term—the calorie—to describe the amount of energy required to increase one gram of water by one degree Celsius.
Water, which is made up of two hydrogen atoms and one oxygen atom, is electronegative. Because one side of an electronegative atom has a partially positive charge and the other has a partially negative charge, it is more likely to attract electrons. The oppositely charged sides are naturally pulled to one other, resulting in a weaker hydrogen bond. That is why water can flow past itself while still bonding with itself—it is continually making and breaking these ties.
These connections are also responsible for liquid water’s high specific heat. Any energy used to heat water is divided equally between breaking the bonds and heating the water. As a result, heating water requires more energy than heating other things.
Why Specific Heat Capacity of Water so High?
The hydrogen bonds explain why water has a high specific heat. The molecules must vibrate in order to raise the temperature of the water due to the numerous connected hydrogen bonds. Because there are so many hydrogen bonds, it takes more energy to vibrate the water molecules and cause them to break.
Similarly, it takes time for hot water to cool down. Temperature falls as heat is released, and the vibrational activity of water molecules slows. The heat emitted balances out the cooling effect of heat loss from liquid water.
Dimensional Formula of Specific Heat
The dimensional formula for specific heat is as follows:
The dimensional formula of heat = [M1L2T−2]
The dimensional formula of m = M1L0K0
The dimensional formula of ΔT = M0L0K1
Step 1: Substitute the dimensional formulas of Q, m, and ΔT in formula
= [M1L2T−2]/[M1L0K0][M0L0K1]
Step 2: Cancel out the common terms:
= M0L2T−2K−1
So, the dimensional formula of specific heat capacity is = M0L2T−2K−1
Specific Heat Capacity of Some Important Material
| Material | Specific Heat Capacity (J/gºC) |
|---|---|
| Al | 0.902 |
| C (graphite) | 0.720 |
| Fe | 0.451 |
| Cu | 0.385 |
| Au | 0.128 |
| NH3 (ammonia) | 4.70 |
| H2O (l) | 4.184 |
| C2H5OH (l) (ethanol) | 2.46 |
| (CH2OH)2 (l) (ethylene glycol, antifreeze) | 2.42 |
| H2O (ice) | 2.06 |
| CCl4 (carbon tetrachloride) | 0.861 |
| CCl2F2 (l) (a chlorofluorocarbon, CFC) | 0.598 |
| Wood | 1.76 |
| Concrete | 0.88 |
| Glass | 0.84 |
| Granite | 0.79 |
Molar Specific Heat
The molar-specific heat of a matter is the amount of heat required to raise the temperature of one mole of a substance by one degree Celsius or one degree Kelvin.
The unit of molar specific heat capacity is J/(mol K) or J/(mol °C) and “c”. Molar Specific Heat can be calculated mathematically using the following formula:
c = Q/(n ΔT)
Q = ncΔT
where,
- Q = the amount of energy required to change temperature by ΔT
- n = the number of moles of the substance
- c = the specific heat of the system
Specific Heat at Constant Pressure and Constant Volume
Specific Heat at Constant Pressure (CP)
The specific heat capacity at constant pressure (CP) is the amount of heat energy necessary to raise a substance’s temperature by one degree Celsius or Kelvin while maintaining constant pressure. The heat energy required to raise the temperature of the substance, as well as the work done by the substance as it expands against the constant pressure, are both included in CP.
Specific Heat at Constant Volume (CV)
The specific heat capacity at constant volume (CV) is the amount of heat energy necessary to raise a substance’s temperature by one degree Celsius or Kelvin while maintaining its volume constant. Because there is no expansion work done against a constant volume, CV merely includes the heat energy required to raise the temperature of the substance.
The Relationship between CP and CV
The manner in which gas is heated influences its behavior, the volume and pressure changes in temperature, and the amount of heat required to raise the temperature of one gram of gas by one degree Celsius. We can heat the gas using different P and V values.
As a result, the specific heat value is almost infinite. If we don’t give a consistent amount of heat, the specific heat of the gas will shift. As a result, we will require a constant specific heat pressure or volume.
CP – CV = n R
where,
CV = heat capacity at a constant volume
CP = heat capacity at constant pressure
R = the molar gas constant
n = the amount of substance
References
- Emmerich Wilhelm & Trevor M. Letcher, Eds., 2010, Heat Capacities: Liquids, Solutions and Vapours, Cambridge, U.K.:Royal Society of Chemistry
- Engel, Yunus A., and Boles, Michael A. (2010) Thermodynamics: An Engineering Approach, 7th Edition, McGraw-Hill
- https://www.vedantu.com/formula/specific-heat-capacity-formula
- Halliday, David; Resnick, Robert (2013). Fundamentals of Physics. Wile
- Yunus A. Cengel and Michael A. Boles, Thermodynamics: An Engineering Approach, 7th Edition, McGraw-Hill,
- https://blog.prepscholar.com/specific-heat-capacity-of-water
- https://testbook.com/blog/specific-heat-capacity/
- https://www.geeksforgeeks.org/specific-heat-capacity/
