Stereochemistry is the branch of science that deals with the structure in three dimensions. Stereochemistry is the study of stereoisomers and covers the entire spectrum of chemical, 3dinorganic, biological, physical, and especially supramolecular chemistry. At least one carbon sp3- hybridized molecule is required to represent a molecule as a three-dimensional object.
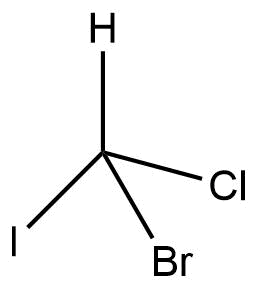
2d structure not suitable for stereochemistry
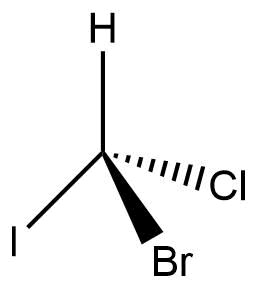
3d structure suitable for stereochemistry
Interesting Science Videos
Objectives of stereochemistry
- To study the various representations of molecules in three dimensions and their effect on physical and biological properties.
- To classify stereoisomers.
- To recognize stereogenic (chiral) centers in molecules.
- To predict, identify and distinguish enetantimers and distereomers.
- To properly define different stereoisomer terminology.
Isomers and isomerism
Isomers are compounds with the same molecular weight and the molecular formula but differ in their properties. This phenomenon is known as isomerism.
Constitutional/ structural isomers
They are the isomers having the same molecular formula but differ in the connectivity of their atoms. There are different types of constitutional isomers. They are as follows:
- Chain isomer: Constitutional isomers are those in which the skeleton of the molecule is organized in different ways to produce various skeletal structures.
- Functional isomer: The isomer having same molecular formula but different funtional groups.
- Positional isomer: Positional isomerism refers to the phenomena when structural isomers differ in the positions of functional groups, substituent atoms, or multiple bonds.
- Metamerism : Metamerism is a phenomenon in which structural isomers differ in the arrangement of alkyl groups surrounding the functional group. An uncommon form of isomerism called metamerism is most frequently found in chemical compounds with divalent atoms joined by alkyl groups.
- Tautomerism: Tautomerism is the tendency of a single chemical compound to exist in two or more interconvertible forms, each of which has a distinct position of proton, electron, or hydrogen.
Steroisomerism
Stereoisomers are isomers that differ in their properties due to the arrangement of atoms and groups in space but have the same molecular formula and molecular weight.
Stereochemistry is typically studied from both static and dynamic perspectives. Static stereochemistry, also known as stereochemistry of molecules, studies the various isomers’ structures, energies, and physical and spectral characteristics. The term “chemistry of reaction” refers to dynamic stereochemistry. It focuses on studying the stereochemical progression of the reaction and interpreting the mechanism of the reaction.
Stereoisomerism is further divided into two classes:
- Conformational isomers
- Configurational isomers
Conformational isomerism
Conformational isomers are stereoisomers formed by rotation about bonds, and they frequently interconvert at room temperature. They are also referred to as conformers, rotational isomers, or rotamers.
Configurational isomers
Stereoisomers that cannot be converted into one another by rotating the molecule around a single bond are known as configurational isomers. These configurational isomers are classified into two types.They are:
- Geometrical isomers
- Optical isomers
Geometrical isomers
When two carbon atoms are connected by a single bond, one carbon atom can rotate over the other. When they are linked by a double bond, rotation is restricted. The carbon atoms become rigid in their position due to the presence of a double bond.
The geometric isomerism is therefore caused by the double bond between the two carbon atoms. Thus, geometrical isomers are the isomers that have the same molecular formula but differ in the arrangement of groups or atoms in space and do not exhibit optical activity.
Compounds with carbon and carbon double-bond are planar. As a result, if a molecule has two different atoms or groups connected to the carbon atom, it will produce two isomers since the two spatial configurations of the groups will be different. so, geometrical isomers can be divided into cis and trans isomers.
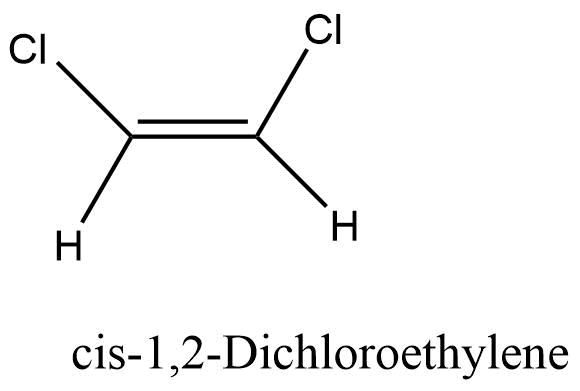
Cis isomer
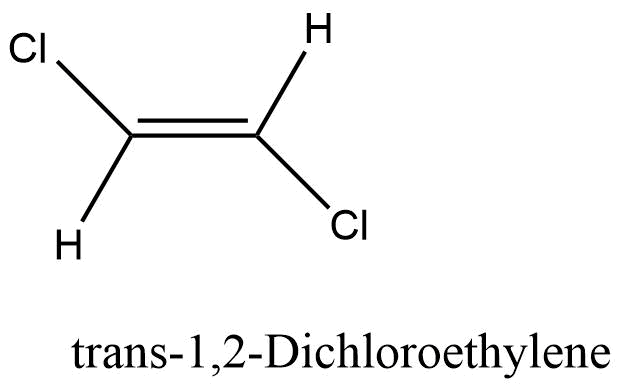
The isomer with the same group on the same side is known as the cis isomer. “Z “is also used to indicate the cis isomer. For example Cis-1,2 Dichloroethylene
Trans isomer
The isomer with the same group on the opposite side is known as the cis isomer. “E” is also used to indicate the cis isomer.
Tetrahedral Carbon
In 1874, Le Bel and Vant Hoff proposed a space theory in which the four valence bonds of carbon are oriented towards the four corners of the tetrahedron, with carbon in the center, and this carbon is known as tetrahedral carbon.
Assymetric carbon
When four valence bonds of carbon atoms are connected with four distinct groups, the carbon atom is known as an asymmetric carbon atom. The molecule containing only one asymmetric carbon atom is always optically active. The essential characteristic of optical activity is the non-superimposability of the two isomeric forms that can exist in a compound containing a single asymmetric carbon atom.
Chiral compounds
The compounds containing a chiral center are called as chiral compounds. They are optically active compounds.
Achiral compounds
Achiral compounds are the compounds which mirror images can be superimposed.
Optical activity
When a substance rotates the plane of polarized light, it is said to be optically active, and this phenomenon is known as optical activity.
Stereocenter
Any atom at which the interaction of two groups results in a stereoisomer is referred to as a stereocenter.
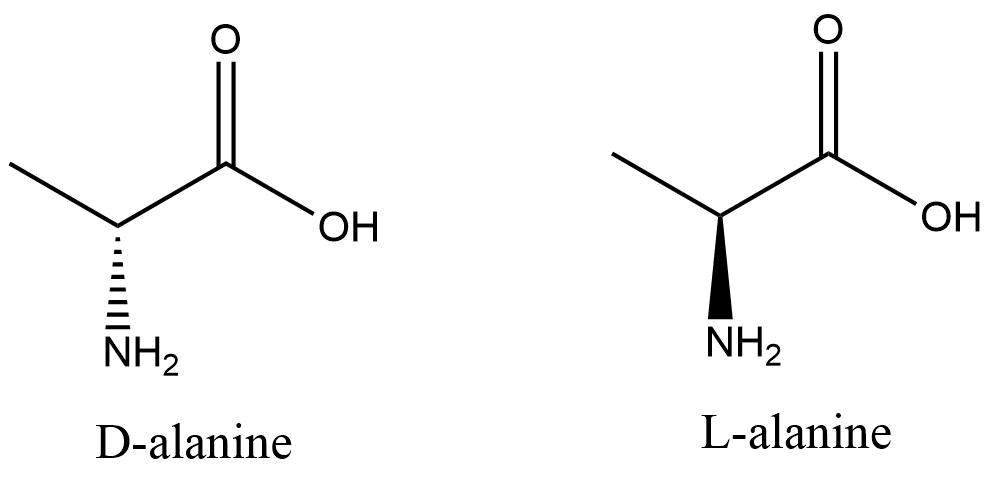
Enantiomers
Enantiomers are stereoisomers that are not superimposable mirror images of one another.
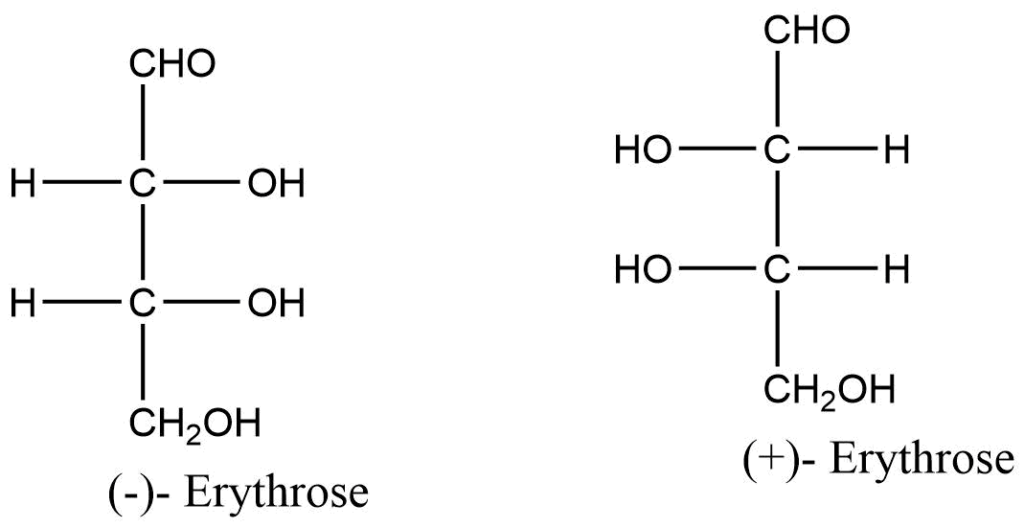
Diasteromers
Compounds with two or more chiral centers that are not mirror images of one another are known as diastereomers.
Epimers
Epimers are diastereomers that have more than one chiral center but differ in absolute configuration at only one chiral center.
Anomers
Anomers are cyclic monosaccharides that differ in the configuration of C-1 or C-2 carbon.
Suggested video
References
- K.R palak,2017, Stereochemistry. Pairavi Prakashan.
- https://www.uou.ac.in/lecturenotes/science/MSCCH-17/CHEMISTRY%20LN%201%20STERIOCHEMISTRY.pdf.
- https://www.vedantu.com/chemistry/stereochemistry.
- https://www.aakash.ac.in/important-concepts/chemistry/isomerism.
- https://www.colby.edu/chemistry/CH241F/Chapter%204.pdf.
