When a substance changes directly from its solid state to its gaseous state, this transition is known as sublimation in the chemical sciences. Crystalline iodine and solidified carbon dioxide are chemicals that sublimate at standard ambient temperatures and atmospheric pressure. However, the vast majority of chemical compounds and elements can exist in all three forms (solid, liquid, and gas) at standard pressures and temperatures. The solid-to-gas transition in such instances necessitates a liquid-to-gas stage.
Interesting Science Videos
What is Sublimation?
Sublimation is the process by which a substance transitions directly from the solid phase to the gaseous phase, bypassing the liquid phase. Sublimation is a physical process that takes place when the pressure of the surrounding atmosphere is insufficient to maintain a substance in its liquid state.
- The process of sublimation can be defined as the opposite of deposition. Deposition, also known as desublimation, refers to the process by which a gas undergoes a direct transformation into a solid state.
- The sublimation of a substance from a solid to a gaseous state is contingent upon its triple point, which refers to the temperature and pressure at which the substance exists in equilibrium as all three states of matter.
- The aforementioned phenomenon denotes an endothermic phase transition, which occurs at a temperature and pressure lower than the substance’s triple point.
- The term “sublimation” specifically pertains to alterations in the physical state of matter, rather than chemical transformations that convert solids into gaseous forms.
- Sublimation is a process utilized for compounds that have the ability to transition directly from a solid to a gaseous state when subjected to heat, without passing through a liquid phase, and in the absence of a volatile source.
- Organic compounds that possess covalent bonds exhibit a greater tendency to sublime in comparison to ionic molecules. This is because the bonds in organic compounds can be broken with relatively low energy requirements.
Characteristics of Sublimation Process
- The process of sublimation is characterized by its endothermic nature.
- Solids undergo direct conversion into gases without passing through a liquid state.
- The term “sublimation” refers to a physical state change and is not utilized to depict a chemical reaction that transforms a solid into a gas.
- When solid ammonium chloride is heated, it dissociates into hydrogen chloride and ammonia. This chemical reaction results in the formation of different products. Hence, it can be concluded that the process is chemical in nature and not sublimation. Candles made of paraffin wax do not undergo sublimation. Instead, they undergo a chemical reaction with oxygen, resulting in the production of carbon dioxide and water vapor as they burn.
- Solid compounds that undergo sublimation typically have weak intermolecular forces. Examples of such compounds include camphor, anthracene, naphthalene, and pyrene.
Examples of Sublimation
Carbon dioxide
Solid carbon dioxide (dry ice) sublimes everywhere along the line below the triple point at pressures and temperatures above the triple point (for example, at 5.1 atmospheres and 56.6 degrees Celsius, for a temperature of 194.65 degrees Kelvin, or 109.30 degrees Fahrenheit), whereas its melting into liquid CO2 can occur anywhere along the solid-liquid line at pressures and temperatures below the triple point).
Naphthalene
Sodium naphthalene, a non-polar chemical compound that is frequently present in pesticides like mothballs, quickly sublimes because it is made up of non-polar molecules and is only held together by the weak van der Waals intermolecular forces. The sublimation point of naphthalene, a solid that sublimes at ambient temperature, is roughly 80 degrees Celsius or 176 degrees Fahrenheit. At low temperatures, such as 1 mmHg at 53 °C, its vapour pressure is sufficient to cause the solid form of naphthalene to evaporate and turn into a gas. If the naphthalene vapours come into touch with chilly surfaces, they will condense into needle-like crystals.
Frost and water
At very low temperatures, water vapor undergoes reverse sublimation, resulting in the formation of ice crystals on surfaces, or “frost.” The substance to be dehydrated is frozen during the freeze-drying process, and its water is then allowed to sublime under reduced pressure.
Iodine
Iodine emits fumes when heated gently. Iodine vapor can reveal hidden fingerprints on paper in forensic science.
Arsenic
The metal industry uses arsenic, a brittle, steel-gray, very poisonous metal. Around 615 °C, high temperatures cause arsenic to sublime as well. Arsenic directly changes from a solid to a gaseous state at ordinary atmospheric pressure.
Cadmium and zinc
Ca and Zn easily sublime, making them unsuitable materials for usage in decreased pressure or vacuum. They are sublimated in sealed containers because they would quickly vanish otherwise.
Applications of Sublimation in Daily Life and Industry
- The forensic sciences can benefit from sublimation. It is possible to use iodine sublimation to reveal forensic evidence on paper.
- A chemist’s approach for purifying volatile chemicals is sublimation. For organic substances, it is highly helpful.
- Naphthalene balls are used in the pesticide business to repel insects and moths.

- Camphor sublimes to produce a pleasant scent in the fragrance industry.
- Dye-sublimation printers have taken the place of inkjet printers. The printouts are usable as soon as they leave the printer since the prints dry more quickly.
- In the textile industry, synthetic materials like polyester are printed using a printing method called dye sublimation.
- Using this method, t-shirts, flags, and banners may all be produced.
- Dye sublimation is used to print a wide range of items at a lower cost, including pens, coffee cups, and bags.

- Space agencies like NASA and ISRO employ this technique to feed people in orbit with high-quality meals. This technique is employed when a food product needs to be stored for an extended period of time.
- The process of freeze-drying is a common use of sublimation in the frozen food sector. When the pressure in the surrounding area is lowered, frozen water in the substance sublimates from the solid to the gas phase.
- We are all familiar with scented perfume tablets that are used to fill the bathroom or wardrobe with a nice scent. They impart a delicate and distinctive smell to your house. They are comprised of 100% organic wax that has been infused with pure essential oils to increase their potency and durability. A perfume tablet can last for three to six months, depending on how it is used. The majority of these tablets have camphor as a key fragrance component. Camphor can sublimate when it’s at room temperature. As a result, these aromatic tablets seem to get smaller with time.

- The majority of celestial objects, including planets, galaxies, and stars, are created by accretion processes. By causing streaming instabilities or promoting the formation of planetary cores, drifting pebbles are known to play a significant part in the core accretion scenario. Additionally, it appears that ice lines of flammable species, like water, are promising locations for this activity. The increased surface energy of the ice at the water ice line encourages coagulation at the water ice line, and the sublimated vapor can spread throughout the disk and settle onto pebbles, allowing for quick development.
- There is no trash created throughout the procedure, making it both secure and environmentally friendly.
Molecular Perspective on Sublimation
From a molecular standpoint, sublimation can be understood as the process by which a solid substance transitions directly into a gas phase without passing through a liquid phase. Understanding the molecular mechanisms underlying sublimation is important for a variety of applications, including the development of new materials and the optimization of industrial processes.
- This phenomenon is driven by the energy input that overcomes the intermolecular forces holding the solid together, allowing the molecules to escape into the gas phase.
- The temperature and pressure conditions at which sublimation occurs are dependent on the specific properties of the substance in question, including its molecular weight, polarity, and crystal structure.
- Similar to other types of phase changes, sublimation is reliant on both kinetic energy and Van der Waal’s forces. We commence with a substance that possesses a definite shape and volume. It is understood that molecules possess a defined arrangement, restricted mobility, and predetermined volume.
- The dominant intermolecular force at play is Van der Waal’s force. The molecular entities are unable to undergo sliding motion relative to each other.
- In gaseous states, the kinetic energy of the particles dominates over Van der Waals forces. The particles exhibit a high degree of mobility within the confines of their respective container.
- In the context of an ideal gas, it is commonly assumed that the collisions between gas particles are characterized by perfect elasticity. The particles do not engage in any form of interaction with each other, and there exists a void space between them. Gases possess the property of occupying the entire volume of their container and exhibiting the ability to undergo expansion and compression.
- Compounds that undergo sublimation typically exhibit weak Van der Waal’s intermolecular forces. Their interactions are predominantly governed by dispersion forces.
Explanation on Carbon dioxide Sublimation
One commonly cited example is that of solid carbon dioxide, also known as dry ice.

- Due to the presence of carbon-oxygen double bonds, it is reasonable to anticipate that carbon dioxide possesses polarity. There exists a significant disparity in electronegativity between the aforementioned pair of chemical elements.
- Carbon dioxide possesses a linear molecular geometry. The cancellation of dipoles is observed. Carbon dioxide molecules are subject to dispersion forces exclusively.
- For carbon dioxide to exist in a solid state, it is necessary to maintain extremely low temperatures. The sublimation temperature of the substance is -78.5°C. That is extremely cold. It is important to note that temperature is defined as the measure of the average kinetic energy of particles within a system. What is the significance of such low temperatures? The experimental results demonstrate the potential for kinetic energy to dominate over dispersion interactions with relative ease. Alternatively, one may choose to elevate the pressure. Dry ice, also known as solid carbon dioxide, can maintain its solid state at room temperature if the atmospheric pressure is above 5.1 atmospheres.
- It is important to note that pressure is defined as the amount of force that is exerted by air particles on a given substance. The molecules exert a force against the adjacent surface molecules, thereby maintaining their cohesion. To contain carbon dioxide, it is necessary to exert a pressure that is more than five times the pressure of our atmosphere.
Therefore, at temperatures exceeding the boiling point, we commence with a solid state. The molecules present on the surface can absorb heat. The temperatures are of such magnitude that they undergo an immediate phase transition into the gaseous state.
Sublimation in Phase Diagram
A phase diagram can be used to represent the phase transitional properties of matter. A phase diagram is a graphical representation that illustrates the changes in the physical state of a substance under different conditions such as temperature and pressure. The graph is a two-dimensional representation that illustrates the correlation between temperature/pressure and the specific state of matter that a substance will assume under those conditions. Water can exist in three different states, namely solid, liquid, or gas, under normal pressure and temperature conditions. In contrast, carbon dioxide can only exist in a gaseous state under normal temperatures and pressures.
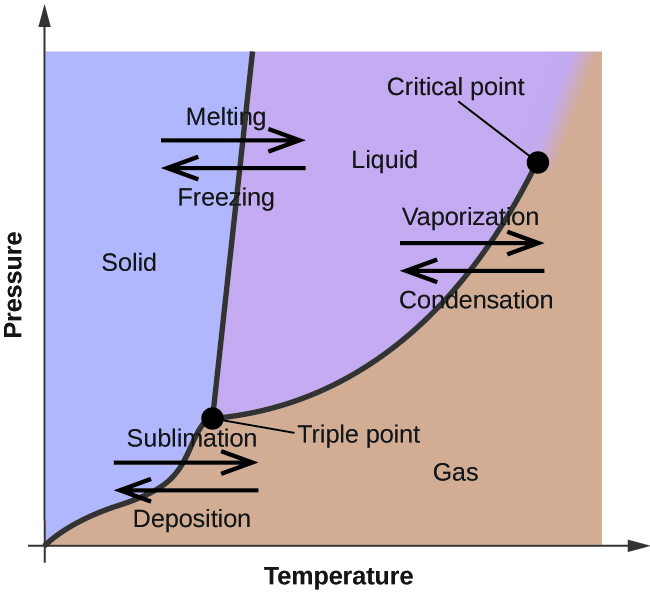
The lines on a phase diagram indicate the points at which a substance undergoes a phase transition from one state to another. In the picture above, the term sublimation refers to any transition that occurs between the two sections that are separated by the bottom line. Under typical conditions of pressure and temperature, the majority of compounds require a transition from a solid to a liquid state before transitioning to a gas. The vapor pressure of a substance can cause common substances like water to sublimate at relatively low temperatures under normal atmospheric pressures.
References
- https://studiousguy.com/sublimation-examples/
- https://www.aakash.ac.in/important-concepts/chemistry/sublimation
- Helmenstine, Anne Marie, Ph.D. “Sublimation Definition (Phase Transition in Chemistry).” ThoughtCo, Feb. 16, 2021, thoughtco.com/definition-of-sublimation-phase-transition-604665.
- https://www.expii.com/t/sublimation-definition-overview-8029
- https://study.com/academy/lesson/what-is-sublimation-in-chemistry-definition-process-examples.html
- https://adesisinc.com/its-sublime-isolating-the-purest-chemical-compounds/
- https://unacademy.com/content/neet-ug/study-material/chemistry/brief-notes-on-sublimation/
- https://sciencetrends.com/what-is-sublimation-in-chemistry/
- https://collegedunia.com/exams/sublimation-chemistry-articleid-786
