Supercritical fluid chromatography (SFC) utilizes a mobile phase consisting of fluid in the supercritical state. As a result of this phenomenon, there are enhancements observed in the isolation of thermolabile compounds, as well as in the separation of compounds with high molecular weight, among other applications. The instrument can be described as a hybrid of a gas chromatograph (GC) and a liquid chromatograph (LC), utilizing either GC capillary columns or high-performance liquid chromatography (HPLC) columns, with a preference for the latter.
The delayed introduction of this technique in the instrumental market, approximately in 1982, has impeded its progress. This can be attributed, in part, to the existence of established normalized methods that have been developed using other conventional chromatography techniques. Furthermore, the complexity and cost associated with the apparatus have resulted in limited interest from instrument manufacturers in developing corresponding analytical instruments.
Interesting Science Videos
What is supercritical fluid?
The supercritical fluid is a phase of matter that exhibits properties that lie between those of a gas and a liquid.
- The formation of this state occurs when a solvent, either in the form of a gas or liquid, is exposed to temperature and pressure conditions that surpass a specific critical point.
- The critical temperature and critical pressure, which are characteristic properties of the solvent, define the specific conditions at which this phenomenon, referred to as “pint,” takes place.
- At this juncture, the solvent will exhibit characteristics that are indicative of both gaseous and liquid phases, while not strictly conforming to either state.
- The behavior of the supercritical fluid in question is contingent upon the prevailing pressure and temperature conditions, determining whether it exhibits characteristics that are similar to a gas or a liquid.
Physical Properties of Supercritical Fluids
Some of the physical properties of supercritical fluids are:
- Density: Supercritical fluids have densities between that of gases and liquids, and are typically more closely associated with liquids. The density of a supercritical fluid rises with increasing pressure (at a fixed temperature) in the supercritical region. Under constant pressure, the density of a substance will decrease as its temperature rises. A supercritical fluid’s ability to dissolve is proportional to its density. Because of their greater density, supercritical fluids make superior transporters in comparison to gases. Analytical methods employing supercritical fluids as solvents rely heavily on density as a result.
- Viscosity: A supercritical fluid has a viscosity that is around one-tenth that of a liquid, making it almost the same as a gas. As a result, supercritical fluids offer less resistance to components passing through them than liquids. Another difference between liquids and supercritical fluids is that the latter’s viscosity is highly sensitive to changes in temperature, whereas the former’s is not. Density, diffusivity, and viscosity are all interconnected. All of them may be impacted in many permutations by the alterations in temperature and pressure. Increases in pressure lead to increases in viscosity, while increases in viscosity lead to decreases in diffusivity.
- Diffusivity: A supercritical fluid’s diffusivity can be 1,000 to 10,000 times lower than that of a gas while being 100 times more than that of a liquid. A solute is expected to exhibit greater diffusivity in a supercritical fluid than it would in a liquid, given that the former has greater diffusivity than the latter. The property of diffusivity, unlike pressure, is proportional to temperature. As pressure is increased, the molecules in a supercritical fluid move closer together, resulting in less diffusion. Supercritical fluids have the potential to be faster carriers for analytical applications because of their increased diffusivity. As a result, chromatography and extraction techniques rely heavily on the use of supercritical fluids.
What is Supercritical Fluid Chromatography?
Supercritical fluid chromatography (SFC) is a separation technology that typically employs supercritical carbon dioxide as the primary mobile phase. Supercritical fluid chromatography (SFC) is advantageous over traditional high-performance liquid chromatography (HPLC) in terms of column back pressure due to the low viscosities and high diffusivities of supercritical fluids. In addition, high ow rates allow for fast analysis, and a longer column allows for a more detailed study. SFC equipment and packed columns designed for SFC have advanced to the point where HPLC-like analyses may be carried out.
- In supercritical fluid chromatography (SFC), the mobile phase is a supercritical fluid.
- The diffusion coefficient of compounds in supercritical fluids is several hundred times larger than in liquids, and their viscosity is less than half that of water. Because of this, SFC maintains its excellent separation efficiency even at high mobile phase flow rates.
- Since SFC uses gas as its mobile phase, it can separate chemicals much more quickly than high-performance liquid chromatography (HPLC), which uses a liquid.
- Supercritical fluids’ density and characteristics fluctuate with pressure and temperature, allowing the separation rate to be customized.
- In addition, the introduction of an auxiliary solvent, such as alcohol, into the supercritical fluid can lead to alterations in the characteristics of the entire mobile phase, thereby allowing for the manipulation of retention time and separation.
- Typically, carbon dioxide is employed as the mobile phase in Supercritical Fluid Chromatography (SFC). Carbon dioxide exhibits similar polarity to hexane, rendering it a viable option for the separation of non-polar substances. Due to this rationale, a column employing silica gel as a packing material exhibits retention characteristics akin to those observed in normal phase chromatography.
- Packed columns can also be employed for reverse-phase chromatography. In this specific instance, a modifier is employed to segregate substances with significant polarity. To achieve the separation of highly polar substances, it is imperative to augment the quantity of modifier that is introduced.
- The introduction of alcohol into carbon dioxide results in an elevation of the critical point of the mixture, surpassing that of carbon dioxide alone. Due to this reason, the process of separation is frequently conducted in subcritical or liquid states, wherein the mobile phase has not attained the supercritical state. Nevertheless, for the sake of convenience, it is still denoted as SFC.
Principle of supercritical fluid chromatography
In analytical chemistry, the separation of pharmaceuticals, herbicides, natural products, and food products is performed using a high-performance liquid chromatography (HLPC) apparatus known as supercritical fluid chromatography (SFC). Supercritical fluids like carbon dioxide, ammonia, water, methanol, n-butane, n-propanol, etc. are used in normal-phase chromatography. Because of its low critical temperature (Tc) and compatibility with the FID detector, carbon dioxide is the optimal fluid for supercritical fluid chromatography.
It was found in 1985 by combining the strengths of HPLC and GLC. Supercritical fluid chromatography is particularly valuable because it enables the separation and identification of chemicals that cannot be separated by high-performance liquid chromatography (HPLC) or gas chromatography (GLC).
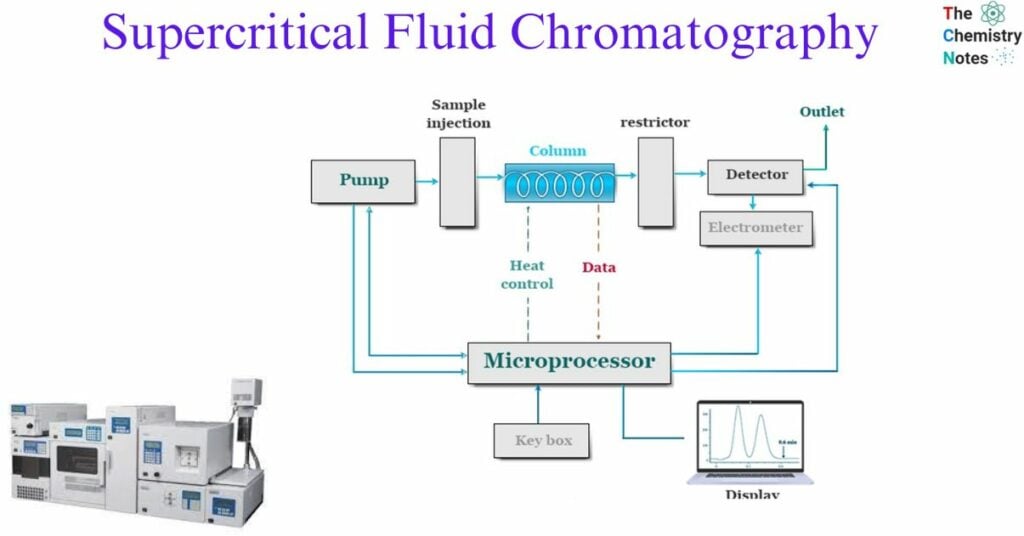
Instrumentation of Supercritical Fluid Chromatography
The important components of supercritical fluid chromatography are:
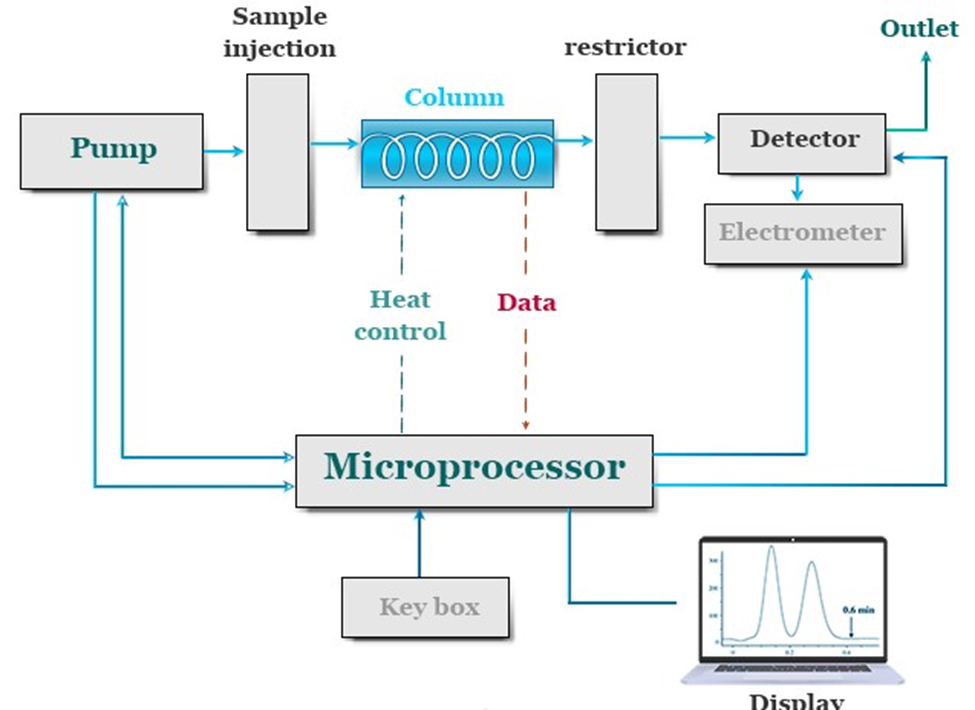
- Pressure regulator: The pressure regulator plays a crucial role as a component within the SFC instrument. The density of the mobile phase is impacted. An increase in density leads to a corresponding increase in the solvent power of the mobile phase. The elution time of the SFC instrument is decreased. As an example, when the pressure of carbon dioxide molecules decreases from 70 to 90 atmospheres, there is a corresponding decrease in elution time from 25 to 5 minutes. Hence, pressure programming plays a crucial role in the fundamental principles of supercritical fluid chromatography.
- Stationary phase: SFC columns exhibit similarities to HPLC columns with respect to their coating materials. The two predominant types of columns employed in Supercritical Fluid Chromatography (SFC) are open-tubular columns and packed columns. Open-tubular columns are commonly favored due to their similarities to fused-silica columns used in High-Performance Liquid Chromatography (HPLC). This particular column is characterized by the presence of an internal coating composed of a stationary phase made up of cross-linked siloxane material. The coating thickness ranges from 0.05 to 1.0 μm. The column’s length exhibits a range spanning from 10 to 20 meters.
- Mobile phase: In supercritical fluid chromatography (SFC), a mobile phase can be made of a large variety of different substances. The mobile phase can be chosen from the solvent groups consisting of inorganic solvents, hydrocarbons, alcohols, ethers, and halides; alternatively, it can be one of the following: acetone, acetonitrile, pyridine, and so on. Because its critical temperature and pressure are so simple to achieve, carbon dioxide is the supercritical fluid that is utilized in SFC more frequently than any other substance. In addition, carbon dioxide has a low cost, is simple to acquire, is unaffected by ultraviolet light, does not contribute to poisoning, and is an effective solvent for non-polar compounds. In addition to carbon dioxide, the following compounds can also be used: ethane, n-butane, N2O, dichlorodifluoromethane, diethyl ether, ammonia, and tetrahydrofuran.
- Detector: One of the most significant benefits of SFC in comparison to HPLC is the extensive selection of detectors. It is possible to use the flame ionization detector (FID), which is typically part of the equipment for GC, to the configuration for SFC. Due to the fact that FID is an extremely sensitive detector, a detector of this kind can provide a positive contribution to the quality of SFC analyses. A mass spectrometer, a UV-visible spectrometer, or an infrared spectrometer can be coupled to an SFC system more easily than an HPLC system can be related to these types of spectrometers. Thermionic detectors and fluorescence emission spectrometers are two examples of additional detectors that can be connected to SFC. These detectors are typically used in conjunction with HPLC.
SFC in chromatographic techniques
SFC has the potential to be useful in many contexts. The main difference is that the selectivity can be changed without changing the eluent’s chemical composition by simply changing the P and T parameters through programming. Due to the low viscosity of the mobile phase, a series of HPLC-type columns can be set up. Lipids and oils, emulsifiers, oligomers, polymers, and molecules with molecular masses of more than 1000 are just some of the substances that can be analyzed by SFC. When compared to HPLC, the speed and efficiency of SFC are far superior. Finally, using supercritical carbon dioxide as a mobile phase makes it easier to couple to other instruments including mass spectrometers, infrared spectrophotometers, and nuclear magnetic resonance (NMR) spectrometers.
Advantages of supercritical fluid chromatography
- The utilization of supercritical fluids in the SFC technique is facilitated by their unique physical properties, which lie between those of liquids and gases. This characteristic allows SFC to effectively integrate the advantageous features of both HPLC and GC. Specifically, the reduced viscosity of supercritical fluids enables SFC to surpass HPLC in terms of speed and efficiency. A decrease in viscosity results in an increase in the velocity of the mobile phase.
- Supercritical fluid chromatography (SFC) enables the analysis of delicate materials that are susceptible to elevated temperatures, owing to the exertion of critical pressure on the substances. The aforementioned materials involve compounds that undergo decomposition at elevated temperatures, as well as materials characterized by low vapor pressure or volatility, such as polymers and complex biological molecules.
- Furthermore, it should be noted that the diffusion of the various components that flow through a supercritical fluid is greater compared to that observed in high-performance liquid chromatography (HPLC). This discrepancy can be attributed to the higher diffusivity exhibited by supercritical fluids in comparison to conventional liquid mobile phases. Consequently, this leads to enhanced distribution within the mobile phase and improved separation.
Application of Supercritical Fluid Chromatography
- This technique is utilized for the purpose of isolating a diverse range of substances, including pharmaceuticals, herbicides, pesticides, and organic food products.
- Supercritical fluid chromatography (SFC) is primarily employed for non-polar compounds due to the limited solubility of polar solutes in carbon dioxide, the most prevalent supercritical fluid mobile phase.
- SFC is a widely employed technique in the petroleum industry for the quantitative analysis of total aromatic content, as well as for the separation of various hydrocarbon compounds.
- The employment of the Supercritical Fluid Chromatography (SFC) technique proves to be particularly advantageous in the process of isolating a range of dimethylpolysiloxane and oligomers. The separation of oligomers in nonionic surfactants, such as triton X-100, can be achieved through the utilization of a carbon dioxide mobile phase, supplemented with a 1% concentration of methanol as a modifier.
- Chiral separations can be conducted on a wide range of pharmaceutical compounds.
- Supercritical fluid chromatography is a highly effective method for the separation of polyaromatic hydrocarbons.
Comparison of SFC with HPLC and GC
- SFC serves as a complementary method to the traditional techniques of gas chromatography (GC) or normal-phase high-performance liquid chromatography (HPLC).
- The migration of the solute occurs due to a distribution mechanism between the nonpolar stationary phase and a moderately polar mobile phase used for elution.
- The solvation capacity of the mobile phase is determined by the temperature and pressure of the supercritical fluid.
- Consequently, by increasing the density of the mobile phase composed of supercritical fluid, it becomes possible to induce the elution of components that are retained within the column.
- The resistance to mass transfer between the stationary and mobile phases is lower in HPLC compared to other methods due to the significantly higher diffusion rate in liquids.
- The smaller C factor in Van Deemter’s equation allows for an increase in the velocity of the mobile phase without a significant decrease in efficiency.
- Additionally, the utilization of GC capillary columns is possible due to the similarity in viscosity between the mobile phase and a gas.
- Nevertheless, due to the placement of the restrictor, the pressure differential across the column alters the distribution coefficients of the compounds as they migrate from the starting point to the endpoint.
- This phenomenon leads to an increase in the width of the peak. Due to this rationale, supercritical fluid chromatography (SFC) is capable of achieving separations at reduced temperatures. However, it is important to note that the efficiencies attained in capillary gas chromatography (GC) cannot be replicated in SFC.
What is Supercritical Fluid Extraction?
Supercritical fluids possess distinct physical properties, such as intermediate values for density, diffusivity, and viscosity, which render them suitable for extraction processes that are unfeasible with either liquids, due to their high density and low diffusivity, or gases, due to their insufficient density for effective extraction and transportation of components.
Complex mixtures with multiple components require an extraction step before being separated by chromatography. In an ideal world, the extraction process would be quick, easy, and cheap. Neither should there be any sample loss or breakdown when the extraction is complete. After the components have been extracted, they should be collected quantitatively. Any byproducts of the extraction process should be minimal in quantity, simple to dispose of, and non-toxic to the environment. Traditional extraction methods frequently fall short of these needs. In this respect, SFE offers a number of benefits over more conventional approaches.
The extraction rate is proportional to the mobile phase’s viscosity and diffusivity. The component to be extracted can freely move through the mobile phase because of its low viscosity and high diffusivity. Supercritical fluids allow the components to be extracted more quickly than with other methods because of their increased diffusivity and lower viscosity compared to regular extraction liquids. So, while traditional methods of extraction could take many hours, SFE can complete the process in about 10-60 minutes.
Changing the temperature and pressure of a supercritical fluid can affect its solubilizing power. In contrast, liquids do not react as strongly to shifts in pressure and temperature. As a result, there is room for improvement in the dissolving capacity of SFE.
Advantages of SFE
Supercritical fluid extraction (SFE) offers numerous advantages.
- Rapid process: The rate at which mass is transferred between a sample matrix and an extraction fluid is influenced by two factors: the diffusion rate of a species in the fluid and the viscosity of the fluid. A higher diffusion rate and lower viscosity result in a greater rate of mass transfer.
- Changing the pressure, and to a lesser extent the temperature, of a supercritical fluid, affects its solvent strength.
- It’s not uncommon for supercritical fluids to be gases at room temperature.
- There exist supercritical fluids that satisfy these criteria while also being inexpensive, nontoxic, and inert.
References
- Taylor, Larry T. (2009). “Supercritical fluid chromatography for the 21st century”. The Journal of Supercritical Fluids. 47 (3): 566–573. doi:10.1016/j.supflu.2008.09.012
- Taylor, Larry T. (2010). “Supercritical Fluid Chromatography”. Analytical Chemistry. 82 (12): 4925–4935. doi:10.1021/ac101194x
- https://jascoinc.com/learning-center/theory/chromatography/introduction-to-sfc/
- https://www.priyamstudycentre.com/2021/11/supercritical-fluid-chromatography.html
- https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Physical_Methods_in_Chemistry_and_Nano_Science_(Barron)/03%3A_Principles_of_Gas_Chromatography/3.03%3A_Basic_Principles_of_Supercritical_Fluid_Chromatography_and_Supercrtical_Fluid_Extraction
