Synthetic polymers, also known as man-made polymers, are polymers that are artificially produced by humans.
Polymers are composed of repetitive structural units called monomers. Polymers, in essence, are substances comprised of extensive, recurring chains of molecules. Each of them possesses distinct characteristics. A polymer is a class of compounds, whether natural or synthetic, characterized by the presence of macromolecules—exceedingly large molecules. These macromolecules are formed through the repetition of basic chemical units known as monomers. The term “polymer” encompasses an indeterminate quantity of monomer units.
When the quantity of monomers reaches a significant magnitude, the resulting compound is occasionally referred to as a high-density polymer. Certain natural polymers consist of a singular variety of monomers. Copolymers, which consist of a combination of two or more distinct monomers, encompass the majority of both natural and synthetic polymers.
Polymers are composed of numerous recurring monomer units that form elongated chains, occasionally exhibiting branching or cross-linking connections between these chains. A polymer can be likened to a necklace composed of numerous small beads, known as monomers. Polymerization, an intricate chemical process, entails the transformation of monomers into polymers. This multifaceted phenomenon encompasses a diverse array of types, each characterized by its own distinct set of properties and mechanisms. Plastic, a term widely used to refer to various synthetic polymer materials, derives its etymology from the Greek word “plastikos,” denoting its inherent ability to be molded or shaped according to desired forms.
Interesting Science Videos
Types of Polymers
There are three different types of polymers that include:
Natural Polymer
- Natural polymers refer to a class of polymers that are derived from natural sources, namely plants, and animals.
- These polymers occur naturally in the environment and possess distinct characteristics that set them apart from their synthetic counterparts.
- There are several other prevalent instances of substances that can be cited, such as protein, which is present in both humans and animals, cellulose and starch, which are found in plants, and rubber, which is obtained through the extraction process from a specific plant source.
Semi-Synthetic Polymer
- Semi-synthetic polymers are derived through deliberate modifications made to natural polymers within a controlled laboratory setting.
- These polymers are synthesized through carefully controlled chemical reactions within a controlled environment, rendering them of significant commercial importance.
- Some examples of materials that utilize sulfur as a binding agent for polymer chains include mature rubber. Additionally, cellulose acetate, which is commonly known as rayon, is another notable example.
Synthetic Polymer
- The manufacturing sector plays a crucial role in meeting the ever-growing demands and evolving preferences of consumers by producing synthetic polymers.
- Polyethylene, a polymer that is becoming more frequently used in packaging materials, and nylon threads, commonly encountered in garments and fishing lines, exemplify two of the most pervasive polymers that permeate our daily existence.
Synthetic Polymer
- Synthetic polymers, also known as man-made polymers, are polymers that are artificially produced by humans. Polymers are composed of repetitive structural units called monomers.
- Polyethylene, a polymer renowned for its simplicity, is composed of ethene or ethylene monomer units. Specifically, the resulting polymer is referred to as high-density polyethylene (HDPE). Synthetic polymers are generated via various types of chemical reactions.
- Polyethylene, an additional polymer, is synthesized through the reproduction of ethylene monomers. The composition of this particular entity consists of an extensive arrangement of elongated chains, potentially encompassing a staggering number of up to 10,000 individual monomers.
- Polyethylene, a material known for its crystalline nature, exhibits the remarkable properties of translucency and thermoplasticity. This implies that when subjected to elevated temperatures, it acquires a malleable consistency, rendering it pliable and easily moldable.
- This versatile material finds application in a wide range of industries, serving as a reliable choice for coverings, packaging solutions, molded components, and the fabrication of bottles and containers. The molecular composition of this substance may comprise a range of 50,000 to 200,000 individual monomers.
- This particular compound finds its application within the textile industry. Certain polymers, such as polystyrene, exhibit glassy characteristics and emit a luminous glow even at ambient temperatures. Additionally, these polymers possess the ability to undergo thermal deformation, making them thermoplastic in nature.
Types of Synthetic Polymers
Despite being the essential building elements of life, synthetic polymer history is still in its formative stages. Throughout the history of polymer science, many important polymers have come about, marking important steps in the field’s growth. These polymers have been painstakingly developed and polished through time and have played a critical role in sculpting the current material world. From the beginning of polymer science to the present, many notable polymers have been introduced, each with its own distinct set of features and applications. These polymers have catapulted the area of polymer science to new heights, transforming different sectors and offering up a world of possibilities. Some of these polymers are discussed here:
Low-Density Polyethylene (LDPE)
- Low-Density Polyethylene (LDPE) polymers are widely recognized as a prevalent category of synthetic organic polymers that are commonly encountered in various aspects of daily existence.
- It is a thermoplastic polymer created from ethylene as a monomer. Low-density polyethylene (LDPE) was among the pioneering polymers to undergo synthesis.
- In 1933, it was synthesized through the utilization of a high-pressure free radical polymerization technique.
- LDPE plastic exhibits several desirable properties, including a melting point that is low, ease of moldability, and buoyancy in water because of its low density.
- LDPE, or low-density polyethylene, is primarily utilized in the production of plastic bags for carrying various items.
- Low-density polyethylene (LDPE) is commonly employed in the production of a wide range of items, including containers, dispensing bottles, wash bottles, tubing, plastic components for computer systems, and various laboratory equipment that is molded.
High-density polyethylene (HDPE)
- High-density polyethylene (HDPE) commonly referred to as “alkathene” or “polythene,” is another thermoplastic polymer made from the monomer ethylene.
- It is a more affordable thermoplastic than LDPE, with a linear structure and little to no branching in contrast. The energy and raw materials required to produce one kilogram of HDPE come from 1.75 kilograms of petroleum.
- Given its limited atmospheric reactivity, it is utilized to create storage containers that can hold a variety of different substances.
- The cost-effectiveness of HDPE renders it a favorable alternative in various applications that necessitate the use of plastic, such as 3D printers, banners, hovercrafts, and even plastic surgery.
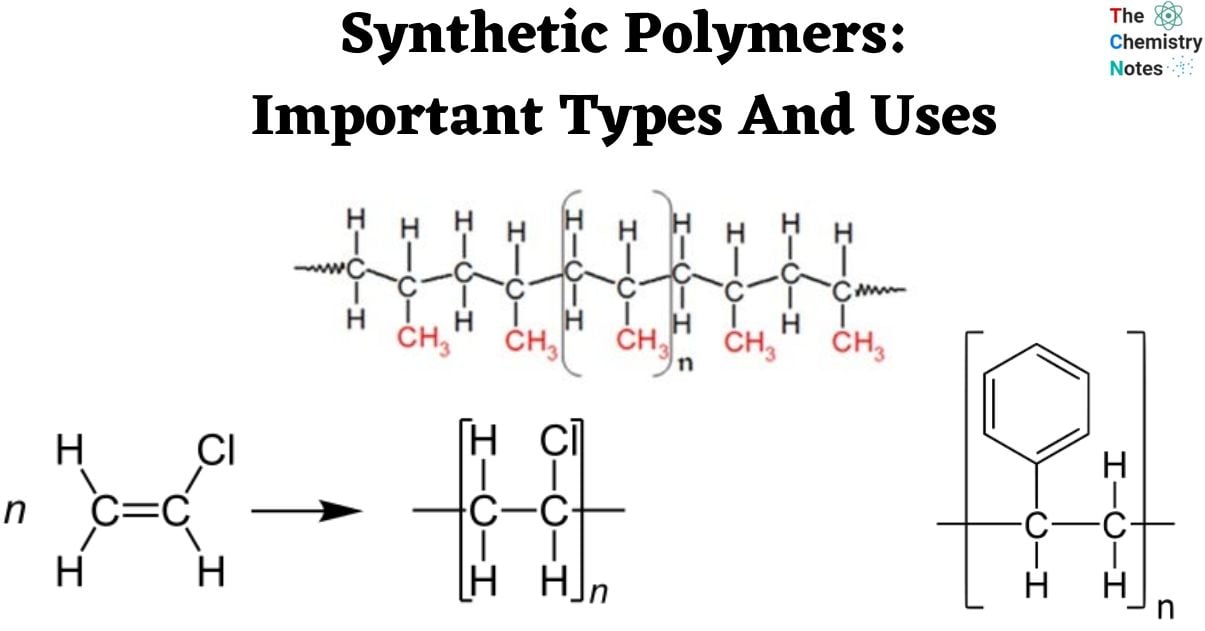
Polypropylene (C3H6)n
- Polypropylene, with its chemical formula (C3H6)n, stands as a paramount example of a thermoplastic polymer that boasts exceptional versatility and cost-effectiveness within the realm of plastics. Propene (or propylene) monomer is polymerized in a chain-growth process to create this polymer. The substance exhibits a rigid and partially crystalline nature.
- It is one of the most reasonably priced plastics on the market right now and is utilized as both a plastic and a fiber in a variety of industries, such as aerospace, furniture assembly, and the production of automobiles.
- There are several uses for it, including packaging and labeling, textiles, stationery, plastic parts and reusable containers of many kinds, laboratory equipment, loudspeakers, automobile parts, and polymer banknotes.
- Polypropylene is a formidable polymer derived from the monomer propylene, exhibiting remarkable resistance to a diverse array of chemical solvents, bases, and acids.
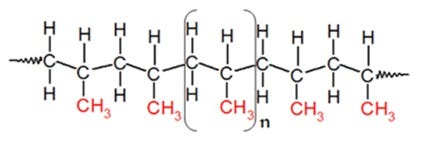
Polyvinyl Chloride (PVC)
- Polyvinyl chloride (PVC) stands as a highly prevalent substance, ranking as the third most widely utilized material in various industries and applications. This particular material is classified as a high-strength thermoplastic, available in two fundamental variants: rigid and flexible. It’s made by polymerizing vinyl chloride monomer.
- It is a brittle, white solid that can be found in granules or powder. PVC is now replacing traditional building materials such as wood, metal, concrete, rubber, ceramics, and others in a number of applications due to its varied qualities such as lightweight, durability, low cost, and ease of processing.
- Construction employs PVC because it’s cheaper and stronger than copper or ductile iron. Phthalates, a common plasticizer, soften them. PVC replaces rubber in clothes, electrical wire insulation, inflatables, and more.
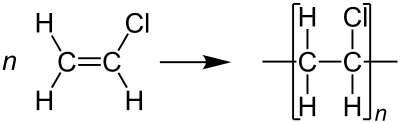
Polystyrene (PS)
- Polystyrene (PS) is a monomer-based aromatic polymer. Depending on manufacture and thermal history, polyester can be amorphous or semi-crystalline.
- In order to create a textile that possesses a combination of desirable characteristics, it is common practice to blend polyester fibers with those derived from natural sources. Polyester synthetic fibers exhibit superior performance compared to their plant-derived counterparts when it comes to withstanding water, wind, and environmental factors.
- The inherent hydrophobic properties of this material render it exceptionally suitable for garments and outerwear intended for utilization in environments characterized by moisture and dampness. The addition of a water-resistant coating to the cloth improves this effect.
- Foamed polystyrene is a versatile material that may be used to make a variety of products, including but not limited to, packaging, insulation, and drinkware.
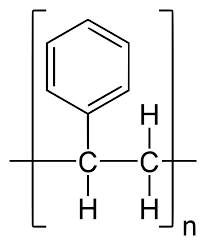
Nylon
- Nylon is a general name for a group of chemicals called polyamides. These are polymers made up of repeating monomer units that are held together by amide bonds.
- Difunctional monomers with equal quantities of amine and carboxylic acid form amides at both ends. The following describes nylon production.
- Nylon is a highly popular polymer that finds extensive usage in various applications. Due to its amide backbone, nylon is even more hydrophilic than the previously mentioned polymers.
- Unlike the exclusive hydrocarbon polymers that make up most plastics, nylon can create hydrogen bonds with water, allowing nylon clothes to absorb water.
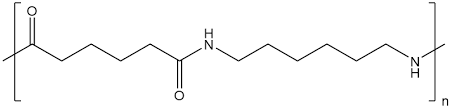
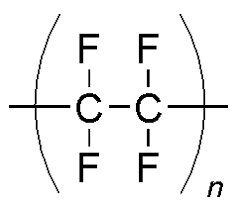
Teflon (PTFE)
- Teflon (polytetrafluoroethylene) (PTFE) is a fluoropolymer with many uses. Carbon and fluorine form PTFE, a solid, high-molecular-weight polymer.
- PTFE’s hydrophobicity prevents it from interacting with water. It’s slippery. Oil and gas, chemical processing, industrial, electrical/electronic, and construction industries use cost-effective PTFE.
- PTFE’s low friction with other compounds makes it a good non-stick coating for pans and other kitchenware. It is often used in barrels and pipes for reactive and corrosive chemicals because it is not very reactive. This is in part because of how strongly carbon and fluorine interact with each other.
Thermoplastic polyurethane (TPU)
- It is a member of a class of polyurethane polymers that possesses several exemplary characteristics, including flexibility, transparency, and resistance to oil, grease, and abrasion.
- A wide variety of unique thermoplastic polyurethanes (TPUs) can be generated through modifications in the ratio, structure, and/or molecular weight of the reactant chemicals. This enables urethane researchers to precisely adjust the polymer’s structure to the material’s desired final properties.
- Thermoplastic polyurethane (TPU) finds diverse applications in various industries, including automotive manufacturing, where it is utilized in instrument panels.
- Moreover, this material is frequently employed in the fabrication of outer casings for mobile electronic devices such as smartphones. Additionally, it is utilized in the construction of protective covers for laptop keyboards.
Categories of Synthetic Polymers
Synthetic polymers can be classified into four distinct categories, namely thermoplastics, thermosets, elastomers, and synthetic fibers.
Thermoplastics
- Thermoplastics typically undergo a phase transition into a molten state when subjected to elevated temperatures, and are generally characterized by a minimal or absent presence of cross-linking.
- Compared to Thermosets, Thermoplastics exhibit greater ease of recyclability and possess the ability to endure heating and reforming processes.
- Linear polymers can be classified as thermoplastic materials. Polymers possess the ability to undergo molding through the application of heat, subsequently solidifying upon cooling.
Thermosets
- Thermosets are characterized by a sufficient degree of crosslinking, which imparts insolubility and resistance to melting upon exposure to heat.
- As a result, these materials are difficult to recycle and are not advocated for environmental sustainability.
- The malleability of these substances is influenced by the application of heat, causing them to conform to specific shapes, and subsequently solidify upon cooling.
- These substances, commonly referred to as resins or synthetic plastics, typically undergo a permanent hardening process when subjected to heat and pressure.
Synthetic Fibers
- Synthetic fibers represent a prevalent category of synthetic polymers. Various synthetic textile fibers are available in the market, comprising both fibers and other materials derived from natural sources, such as rayon, cellulose-based acetate, and regenerated protein fibers sourced from zein.
- They could even be entirely synthetic fibers like nylon or acrylic.
Elastomers
- The elastic characteristics of natural rubber are found in elastomers, which are polymers. The materials exhibit a degree of light cross-linking and lack a well-defined crystalline structure. The glass transition temperature of the material in question is lower than the ambient temperature.
- The phenomenon of crosslinks effectively inhibits any form of irreversible flow. Above the glass transition, the chains’ pliability increases dramatically, and even a moderate force causes considerable distortion. Therefore, it is imperative to exercise due diligence when dealing with elastomers.
- One possible conceptualization of them involves envisioning them as an exceptionally large macroscopic molecule. The intermolecular forces among the polymer chains exhibit relatively lower strength.
Drawbacks of Synthetic Polymers
- Chemical inertness, or resistance to several types of chemical breakdown, is one of many synthetic polymers’ most desired properties.
- The aforementioned characteristic also implies that they possess a prolonged lifespan even after being discarded, thereby posing significant harm to the environment.
- Consequently, it is imperative that appropriate measures are taken to address this issue. Improper disposal of such solid polymer compounds can give rise to a multitude of complications.
References
- https://www.britannica.com/science/polymer/Synthetic-polymers
- https://www.vedantu.com/chemistry/synthetic-polymers
- https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Organic_Chemistry_(Morsch_et_al.)/Chapter_31%3A_Synthetic_Polymers
- https://study.com/learn/lesson/synthetic-polymers-overview-examples.html
- https://uen.pressbooks.pub/introductorychemistry/chapter/synthetic-organic-polymers/
