Tautomerism is the existence of two or more chemical compounds that are easily interconvertible, frequently by simply exchanging one hydrogen atom for another between two other atoms, to either of which it forms a covalent connection. Tautomerism refers to the rapid interconversion of chemical compounds’ structural isomers (constitutional isomers).
Tautomerism is a phenomenon in which a single chemical molecule tends to exist in two or more interconvertible forms that differ according to the relative position of one atomic nucleus, in general, hydrogen. The two structures are referred to as tautomers. These isomer structures often differ simply in the number of electrons and protons. They are also present in dynamic equilibrium.
In the field of chemistry, tautomerism is the most commonly used term. There are various varieties of tautomerism, the most common of which is keto-enol tautomerism. One tautomer exists as a ketone in this kind, whereas the other exists as an enol.
Tautomerism can be used to characterize the reversible interconversions of structural isomers in all cases under normal circumstances, as a result of which they cannot be isolated individually under normal conditions and can only be separated under particular circumstances. They only differ in a. electron dispersion and the position of a relatively mobile atom or group. The two forms are referred to as tautomers or tautomerides. Under normal conditions, two tautomers stay in an equation state, however, this equation can be displaced by temporary acid, alkali, and solvents.
Interesting Science Videos
Conditions of tautomerism
Tautomerization is a chemical reaction that occurs when a catalyst is present.
2. Acid Catalyst: Protonation occurs and the cation is delocalized in the case of an acid catalyst. The deprotonation then occurs at the cation’s neighboring site.
3. Basic Catalyst: In the case of a base catalyst, rather than cation delocalization, anion delocalization occurs first, followed by protonation to a new position of the anion.
Features of Tautomerism
Tautomerism occurs in compounds with polar molecules and weakly acidic functional groups.
It entails a shift in the location of an atom.
It has no effect on bond length or other characteristics.
Tautomerism can arise in both planar and non-planar molecules.
There is atomic mobility involving alpha hydrogen atoms.
Because the compounds are distinct, they may be separated and isolated.
The structures of tautomeric forms differ.
Tautomer compounds are in equilibrium with one another.
Tautomerism does not reduce the energy of the molecules and thus stabilizes them.
Types of Tautomerism
Emil Erlenmeyer, a physicist, devised a rule for Tautomerism in the 1880s. He was one of the first to investigate keto-enol Tautomerism. According to this rule, the hydroxyl group in all alcohols directly links to a double-bonded carbon atom and creates aldehydes or ketones. This was related to the increased stability of the keto form. There are different types of Tautomerism. Some of them are as follows:
Prototropy
The most prevalent type of tautomerism is prototropy, which refers to the translocation of a hydrogen atom. The acid-base behavior of the molecule causes this form of tautomerism. In this case, the only difference between the two forms is the position of a proton. This structure will have the same number of charges as well as the same empirical formula.
So, prototropic tautomers are collections of isomeric protonation states that all have the same empirical formula and total charge. Tautomerization reactions are catalyzed by:
Basic Catalyst:
1. Deprotonation.
2. The formation of a delocalized anion (for example, an enolate).
3. protonation at a different anion site.
Acid Catalyst:
1. Protonation
2. Delocalized cation formation
3. Deprotonation at a separate location near the cation.
Annular Tautomerism
If a proton occupies two or more places in a heterocyclic system, this is referred to as annular Tautomerism. For example: 1H-imidazole and 3H-imidazole.
Ring-Chain Tautomerism
Because of the delocalization of protons in tautomerism, if an open structure is changed to a ring structure, such a tautomer is known as a ring-chain tautomer. Glucose is an example of a ring-chain tautomer.
So, ring-chain tautomerism occurs when the movement of a proton is followed by the opening or closure of a ring. This kind is found in carbohydrates, but it can also be found in other compounds like warfarin.
Valence Tautomerism
Valence tautomerism is a type of tautomerism in which single and double bonds are continuously formed and broken in the compound without any migration of groups or atoms. It occurs quickly and differs from the preceding kind of tautomerism.
However, neither the canonical resonance structure nor the mesomers change in this tautomerism, only the geometrical structure does.
Tautomerism in carbonyl compounds
Keto enol tautomerism
Keto-enol tautomerism: Only keto forms exist:
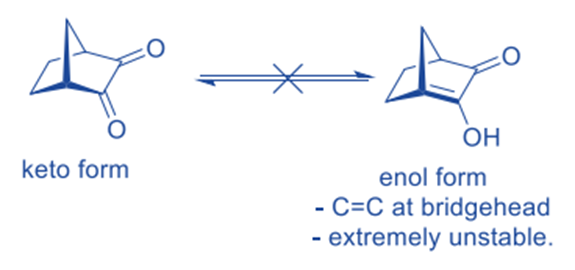
In keto-enol tautomerism, it is observed that the keto form is far more stable than its enol form and hence dominates the tautomeric equilibrium.
With the exception of a few unusual circumstances where certain properties (such as H-bonding, prolonged conjugation, steric crowding, and so on) stabilize the enol forms, most carbonyl compounds reside nearly entirely in the keto form, with just trace amounts of enol content.
Phenol-keto tautomerism
On the other type of the keto-enol tautomerism: Enol forms that are nearly exclusive: Phenols
There are also compounds where the enol is much more stable and there is rarely any keto form to be found. Aromatic enols, often known as phenols, are an example of such a class of chemicals.
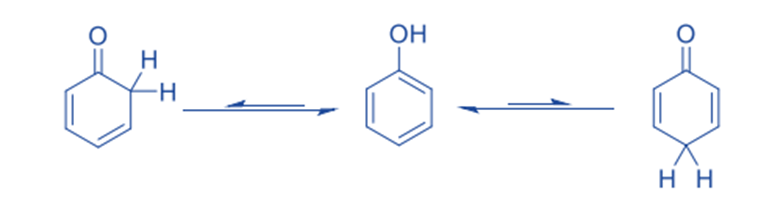
Let us discuss why the enolic form is so prevalent in this case. The following energy variables favor the keto and enols forms:
i) In terms of bond energies, the keto forms enjoy about 15 kcal/mol stabilization
ii) for keto, there is extended conjugation providing an extra 5 kcal/mol stability; and for keto-2, there is conjugation between and C=C, but it is also a cross-conjugated system.
iii) The aromatic enol form has a high stabilizing energy of 36 kcal/mol.
iii) the enol form is aromatic and enjoys large stabilization energy of 36 kcal/mol. This energy gains more than compensates for the energy expense incurred by converting the enol to either of the two non-aromatic keto forms. According to our basic (and imprecise) calculations, the phenol form is at least 16 kcal/mol more stable than any of the keto forms.
This is also why the two keto forms are so acidic; the phenoxide ion, their conjugate basic, is aromatic. Thus, proton departure is associated with a significant decrease in free energy.
The presence of phenols in (nearly) fully enolic form is due to aromatic stabilization, which compensates for the favorable bond energy of the keto form.
Based on the discussion thus far, we may conclude that in order to increase the population of the keto form in phenol-keto tautomerism of phenols, additional factors that lower the difference between enolization and aromatic—conjugation energies must be present.
Factors leading to increased keto content in Phenol-keto tautomerism
Factors that lead to increased keto content in phenol-keto tautomerism include:
- Polyhydroxy monocyclic phenols: The addition of additional hydroxy groups to the phenolic ring aids in the establishment of a ketonic character because the energy produced by repeated keto group formation compensates for the loss of resonance stability.
- an increase in the number of hydroxy groups leads to the equalization of the aromatic conjugation energy to the total enolization energy of several.
- The energy of aromatic conjugation is equalized to the sum of the enolization energies as the number of hydroxy groups increases.
- carbonyl groups; in other words, as we pile more hydroxyl groups onto the aromatic ring, and at appropriate positions so that all of them can simultaneously move over to the keto forms, the bond energy that comes from the keto form may compete with the aromatic stability of the enol.
- one or more aryl rings annulated with the phenolic cycle decrease the total aromatic conjugation energy; because there is a diminishing reactivity as we fuse aromatic rings together.
- bulky groups found in the ortho-positions of phenol produce a steric barrier that stabilizes the quinonoid structure.
Factors affecting keto-enol tautomerization
Steric effects
The amount of enol content may rise or decrease as a result of steric interactions that result in the presence of enol or keto form. When the molecules contain two or more bulky groups, the keto-enol equilibrium shifts predominantly to the enol form due to steric hindrance destabilizing the keto form.
Effect of solvent
The rate of enolization is highly influenced by the solvent (the enol form is less polar, whereas the keto form is more polar). Enol concentration of ethyl acetoacetate in water is 0.4% and 19.8% in toluene. Toluene is a non-polar solvent, whereas water is a polar solvent. As a result of intermolecular H-bonding with the carbonyl, water reduces the enol concentration, making this group less available for intramolecular H-bonding.
Effect of temperature
As the temperature rises, the enol content decreases.
Hydrogen Bonding
Enols have a highly polarized O-H bond; the hydrogen has a partial positive charge and is capable of hydrogen bonding. If a Lewis base (for example, the oxygen of a carbonyl) is close, an intramolecular hydrogen bond can develop, stabilizing the enol form.
Tautomerism in Non-Carbonyl Compounds
Nitro-aci-nitro tautomerism
This type of tautomerism is observed in both primary and secondary nitro compounds. Nitroalkanes (excluding tertiary ones) lose a proton from the a-carbon when exposed to a base. The conjugate base, nitronate anion (similar to enolate), is resonance-stabilized and concentrates most of the excess electron density on the more electronegative O-atom.
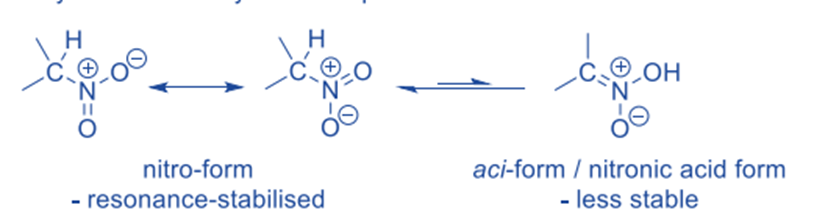
The presence of resonance stabilization in the nitro form means that it is more stable than the aci-form and hence dominates this equilibrium.
Nitroso-oxime tautomerism
Nitroso compounds can tautomerize into oximes by transferring a proton from carbon to oxygen. This process is identical to carbonyl enolization, except that the N=O group replaces the C=O group. Because the oxime form has stronger bonds, the tautomeric equilibrium favors it.

Tautomerism in diazoamino compounds
This tautomeric transformation is a degenerate tautomerism, meaning that the molecule transforms “onto itself” for a symmetrical diazo compound like diazoaminobenzene. In that circumstance, we are unable to tell the difference between the two tautomers. We can see the tautomeric alteration if we use an unsymmetrical diazoamino molecule or replace one of the terminal nitrogens of the triad with an isotope.
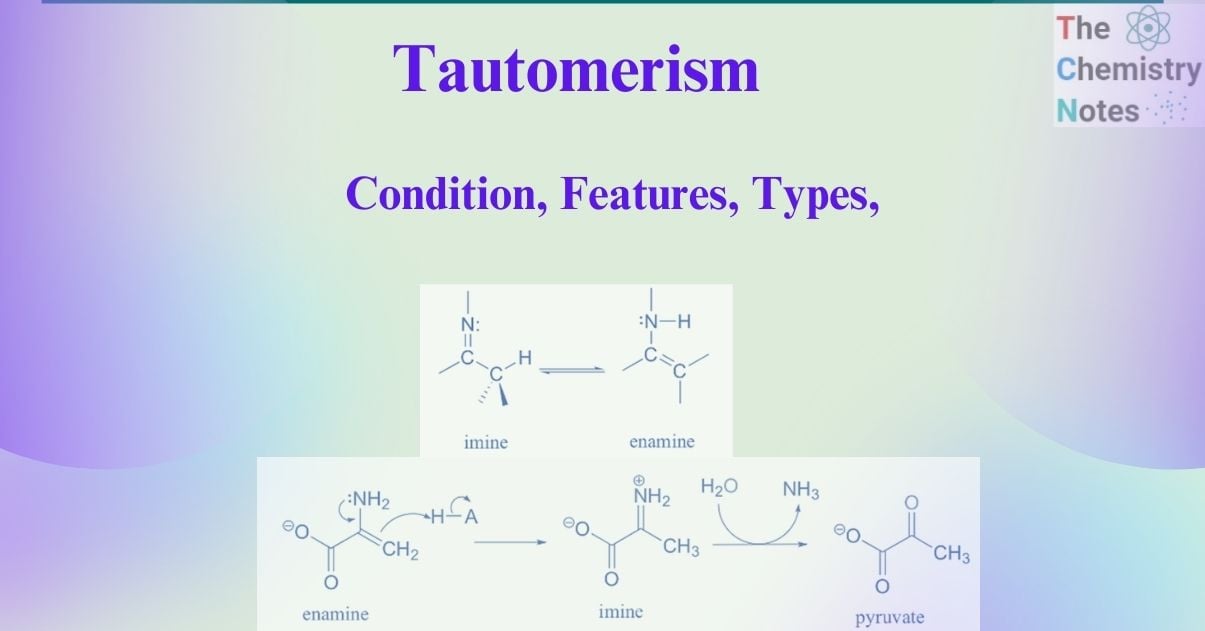
Imine-enamine tautomerism
The nitrogen analog of keto-enol tautomerism is imine-enamine tautomerism.The imine form is equivalent to the keto form, while the enamine (ene+amine) form is equivalent to the enol form. The equilibrium between imines, commonly referred to as Schiff bases and enamines, the nitrogen-based counterparts of enols, is another typical tautomeric connection in biological organic chemistry.
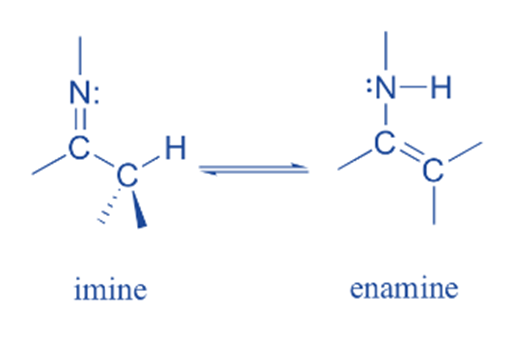
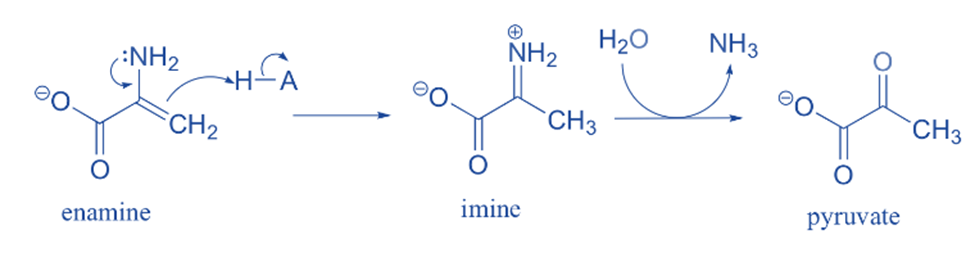
Pyruvate is formed due to the common imine-enamine tautomerism. For example, the breakdown of serine includes an enamine to imine tautomerization phase followed by imine hydrolysis to generate pyruvate.
Difference between Tautomerism and Resonance
1. Tautomerism is frequently associated with the breaking and formation of bonds and bonds. Only the nonbonding electrons and π electrons move during resonance, leaving the σ framework unaffected.
2. During Tautomerism, there is a shift in the location of atoms. There is no atomic movement in the molecule during resonance.
3. Tautomerism may entail an alternation in atom hybridization, resulting in a change in molecule form. There is no change in the hybridization or shape of the molecule during resonance.
4. The two tautomeric forms stay in equilibrium, and a change in the situation can cause them to shift in either way. The tautomers are thus actual structures, whereas the resonance structures are imaginary.
References
1. https://tmv.ac.in/ematerial/chemistry/sat/SEM%20II%20Tautumerism.pdf
2. https://application.wiley-vch.de/books/sample/3527332944_c01.pdf
3. Smith M. & March J. (2001). March’s advanced organic chemistry : reactions mechanisms and structure (5th ed.). Wiley.
4. https://byjus.com/jee/tautomerism/.
5. https://www.britannica.com/science/tautomerism.
6. Morrison R. T. & Boyd R. N. (1983). Organic chemistry (4th ed.). Allyn and Bacon.
