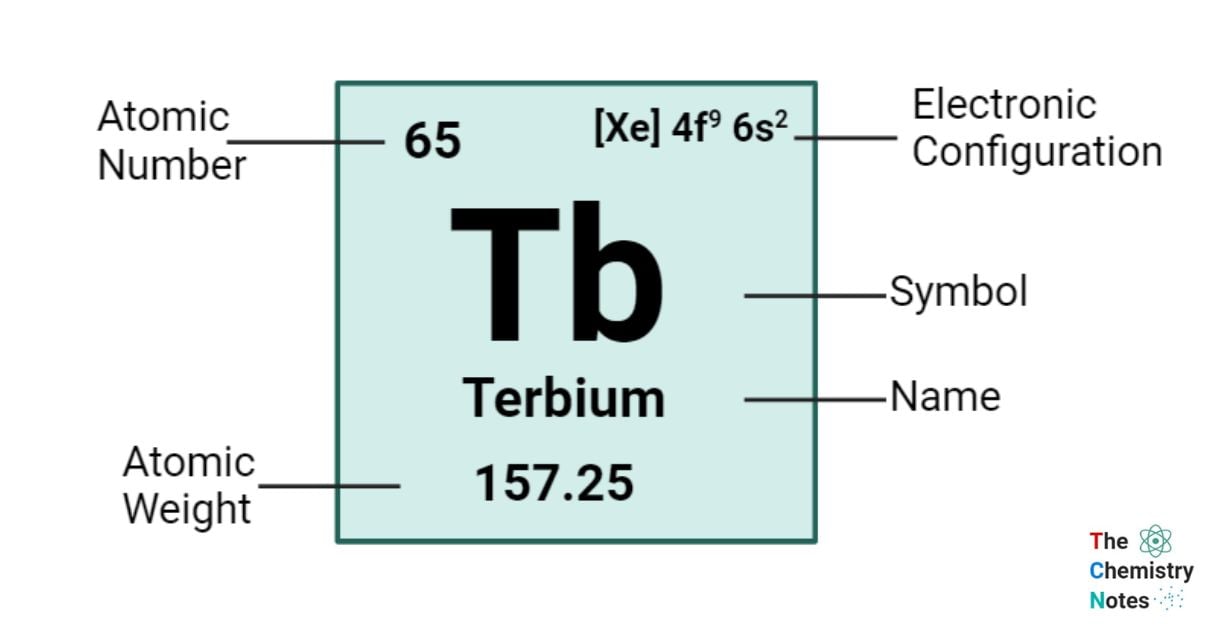
Terbium is a chemical element with an atomic number of 65 and is represented by the symbol ‘Tb’ in the periodic table. It is hard and silvery in appearance and classified as rare earth metal and belongs to the f-block of the lanthanide group of the periodic table. Terbium, like other rare-earth elements and lanthanides, is frequently found in its +3 oxidation state. Terbium, like cerium and praseodymium, exhibits the capability to assume a +4 oxidation state. It is important to note that the +4 oxidation state of terbium becomes unstable in the presence of water. Terbium exhibits a range of oxidation states, encompassing 0, +1, and +2.
Terbium is not naturally occurring in its natural state, however, it can be encountered in various minerals, primarily monazite, xenotime, and euxenite.
Interesting Science Videos
History of Terbium
- Gadolinite, a mineral composed of (Ce, La, Nd, and Y)2FeBe2Si2O10, was initially found in a quarry located in Ytterby, Sweden. This mineral has served as a significant reservoir for numerous rare earth elements.
- In the year 1843, Carl Gustaf Mosander successfully conducted the separation of the mineral gadolinite, resulting in the production of three distinct substances. These substances were subsequently named Terbia, Erbia, and Yttria.
- In 1877, a reversal of nomenclature occurred in the scientific community, wherein the names of terbia and erbia were interchanged. The confusion that resulted from the striking similarities between the characteristics and names of these two substances was what led to this decision.
- Mosander made the discovery of the rare earth elements erbium and terbium based on the analysis of the two aforementioned materials.
- Subsequently, extensive fractional crystallization procedures were conducted by the esteemed French chemist George Urbain to effectuate the separation of rare earth elements, culminating in the acquisition of terbium’s spectral data.
Occurrence of Terbium
- Terbium does not occur naturally in its elemental form; however, it can be found in various minerals, predominantly monazite, xenotime, and euxenite.
- Terbium, along with other rare earth elements, can be commonly discovered in various minerals. For instance, monazite (Ce, La, Th, Nd, Y)PO4 contains terbium in concentrations of up to 0.03%. Additionally, terbium can be found in xenotime (YPO4) and euxenite (Y, Ca, Er, La, Ce, U, Th)(Nb, Ta, Ti)2O6, where the latter mineral can contain 1% or more terbium.
- From a commercial standpoint, the production of this substance involves a complex ion exchange handle utilizing monazite as the source material. The metallic element can be acquired through the reduction of its anhydrous fluoride or chloride using calcium metal within an argon environment.
- Terbium exhibits a total of 26 isotopes, each possessing a distinct half-life, ranging from mass numbers 140 to 165. Terbium in its natural state is composed solely of its stable isotope, 159Tb.
Isotopes of Terbium
Terbium, in its native form, is exclusively comprised of its stable isotope, 159Tb.
Naturally Occurring Isotopes of Terbium
| Isotopes | Natural Abundance (atom %) |
|---|---|
| 159Tb | 100 |
Elemental Properties of Terbium
| Electronic Configuration | [Xe] 4f9 6s2 |
| Atomic Number | 65 |
| Atomic Weight | 158.9254 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | Lanthanides, 6, f-block |
| Density | 8.27 g/cm3 at 20 °C |
| Appearance | silvery-white |
| Van der Waals radius | unknown |
| Electron shells | 2, 8, 18, 27, 8, 2 |
| Electrons | 65 |
| Protons | 65 |
| Neutrons in the most abundant isotope | 94 |
Physical Properties of Terbium

- Terbium has an atomic number of 65 and is a silvery-white rare earth metal. It has a melting point of 1356°C (2473°F) and a boiling point of 3123°C (5653 °F).
- Tb has a solid phase density of 8.23 g/cm3 and a liquid or molten phase density of 7.65 g/cm3.
- It is malleable which means it can be easily beaten into thin sheets without any cleavage.
- It is ductile which means it is possible to draw thin wires from it without breaking.
- Terbium is known to exhibit two distinct crystal allotropic forms, with a transition temperature occurring at 1289°C.
- Terbium exhibits a straightforward ferromagnetic ordering when its temperature is less than 219K (-54.15 °C). In the temperature range of 219-230 Kelvin [-54.15 -(-43.15) °C] a transition occurs in the magnetic ordering, leading to a helical-antiferromagnetic arrangement. Once the temperature exceeds 230 Kelvin (-43.15 °C), the arrangement transitions into a state of disordered paramagnetism.
| Color/physical appearance | metallic, silvery-white |
| Melting point/freezing point | 1629 K (1356 °C, 2473 °F) |
| Boiling point | 3396 K (3123 °C, 5653 °F) |
| Density | 8.23 g cm-3 at 20° |
| Malleability | Yes |
| Ductility | Yes |
| Electronegativity | 1.2 (Pauling Scale) |
Chemical Properties of Terbium
- Terbium readily undergoes oxidation in the presence of air, resulting in the formation of a non-stoichiometric compound known as terbium (III, IV) oxide.
- The element terbium undergoes a chemical reaction with water, resulting in the formation of a hydroxide compound.
- Terbium exhibits reactivity with halogens, resulting in the formation of trihalides corresponding to the respective halogen.
- Similar to all elements in the lanthanide series, compounds including terbium exhibit an oxidation state of +3. Nevertheless, the trivalent terbium species present in a strongly alkaline aqueous solution can undergo a conversion to tetravalent terbium species through the application of ozone.
- Terbium demonstrates the capacity to form binary compounds with carbon, nitrogen, phosphorus, sulfur, boron, selenium, silicon, and arsenic under conditions of elevated temperatures.
Chemical Reaction of Terbium
- The Reaction of Terbium With Air
Terbium metal undergoes a gradual process of tarnishing when exposed to the atmosphere. In addition, it readily combusts to produce terbium oxide, which can be represented by the approximate chemical formula Tb4O7.
8 Tb + 7 O2 → 2 Tb4O7
- The Reaction of Terbium With Water
Terbium exhibits a sluggish reaction when exposed to cold water, but demonstrates a rapid reaction when in contact with hot water. This reaction results in the formation of terbium (III) hydroxide, denoted as Tb(OH)3, along with the liberation of hydrogen gas, represented as H2.
2 Tb (s) + 6 H2O (g) → 2 Tb(OH)3 (aq) + 3 H2 (g)
- The Reaction of Terbium With Halogens
The element terbium exhibits a propensity to engage in chemical reactions with various halogens, resulting in the formation of terbium (III) halides.
The chemical reaction between terbium metal and fluorine gas (F2) results in the formation of terbium (III) fluoride, denoted as TbF3.
2 Tb (s) + 3 F2 (g) → 2 TbF3 (s) [white]
The chemical reaction between terbium metal and chlorine gas (Cl2) results in the formation of terbium (III) chloride, denoted as TbCl3.
2 Tb (s) + 3 Cl2 (g) → 2 TbCl3 (s) [white]
The chemical reaction between terbium metal and bromine (Br2) results in the formation of terbium (III) bromide, denoted as TbBr3.
2 Tb (s) + 3 Br2 (g) → 2 TbBr3 (s) [white]
The chemical reaction between terbium metal and iodine represented as I2, results in the formation of terbium (III) iodide, denoted as TbI3.
2 Tb (s) + 3 I2 (g) → 2 TbI3 (s) [yellow]
- The Reaction of Terbium With Acid
Terbium metal readily undergoes dissolution in dilute sulphuric acid, resulting in the formation of solutions that contain the aquated Tb (III) ion, which exhibits a very pale pink color. Additionally, this reaction produces hydrogen gas, denoted as H2. The existence of Tb3+(aq) as the complex ion [Tb(OH2)9]3+ is highly probable.
2 Tb (s) + 3 H2SO4 (aq) → 2 Tb3+ (aq) + 3 SO42− (aq) + 3 H2 (g)
Uses of Terbium
Numerous significant applications exist for terbium and its compounds some of which are discussed here:
Used As Dopants
Terbium is generally employed as a dopant in various solid-state gadgets such as calcium tungsten, calcium fluoride, and strontium molybdate. It is also utilized as a crystal stabilizer in high-temperature fuel cells, often in combination with ZrO2.
Used In Alloys
Terfenol-D is a specialized alloy composed of terbium that exhibits the unique property of expanding or contracting when subjected to the influence of a magnetic field. This alloy possesses the greatest magnetostriction among all known alloys. Terbium finds applications in the manufacturing of electronic devices and is also utilized in the creation of alloys. Terbium, an essential element in Terfenol-D, finds applications in various fields such as actuator technology, naval sonar systems, sensors, and magneto-mechanical devices. Notably, it was first commercially utilized in the SoundBug device.
Used As Luminescent
Terbium oxide finds application in the production of green phosphors utilized in fluorescent lamps and color television tubes. Sodium terbium borate finds application in solid-state devices. Terbium’s remarkable fluorescence properties render it suitable for utilization as a probe in the field of biochemistry, wherein it exhibits certain similarities to calcium in terms of its behavior.
Used Against Counterfeit
Euro banknotes employ the utilization of rare earth chemistry as a means to effectively counteract counterfeit activities. When a euro is exposed to ultraviolet (UV) light, it exhibits green fluorescence due to the presence of terbium (Tb3+), red fluorescence due to europium (Eu3+), and blue fluorescence due to thulium (Tm3+).
Used In Magnets
Hybrid vehicle engines are equipped with electric motors, which rely on the fundamental principles of magnetism. These magnets must maintain their magnetic properties even under elevated temperatures. The production of such magnets is achieved by alloying neodymium with terbium and dysprosium. Moreover, these magnets find application in the electric motors employed in wind turbines, which are known to generate elevated temperatures.
Used In Fuel Cells
Terbium finds application in the production of fuel cells. A fuel cell is defined as any system that utilizes chemical reactions to generate electrical energy. Fuel cells will likely experience a significant increase in their utilization as a prominent electricity generation technology in the forthcoming years. Terbium fuel cells demonstrate efficient performance under elevated temperatures.
Miscellaneous Applications
- Terbium is employed for endospore detection. Endospores are resilient, quiescent, and non-reproductive structures that are synthesized by certain bacteria belonging to the phylum Firmicutes.
- The application of this technology facilitates the production of medical x-ray images of superior quality, while concurrently decreasing the necessary exposure duration. As a result, this technological progress contributes to the improvement of safety during the implementation of the procedure.
- Terbium salts, such as terbium acetate, find application in specialized laser devices.
Health Effects of Terbium
- Terbium does not possess any discernible biological function and may exhibit mild toxicity when orally consumed.
- The direct contact of terbium powder and compound with both eyes and the skin can cause significant irritation.
- A detailed investigation of its toxicity has not been conducted.
Environmental Effects of Terbium
Currently, there is a lack of knowledge regarding the potential environmental impacts of terbium. It appears that animals and plants are not significantly impacted.
Video on Terbium
References
- https://www.rsc.org/periodic-table/element/65/terbium
- https://www.britannica.com/science/terbium
- https://www.lenntech.com/periodic/elements/tb.htm
- https://pubchem.ncbi.nlm.nih.gov/element/Terbium
- https://www.sciencedirect.com/topics/materials-science/terbium
- https://www.chemicool.com/elements/terbium.html

