Acids can be either organic or inorganic, and they can also be particularly strong or weak. They frequently arise on their own. As an illustration, hydrochloric acid can be discovered in the stomachs of mammals, including humans. Citric acid, present in citrus fruits like lemons and oranges, is an example of an acid that plants naturally produce.
Acids are solutions that are capable of corroding and reacting with a variety of materials, particularly metals. They undergo neutralization reactions with bases, which result in the formation of salts.
Acids are frequently employed in scientific research as both reagents and analytical solutions, particularly in the context of titrations. They are also essential in a wide variety of industrial processes, including the refining of petroleum, the creation of fertilizer, and the manufacturing of pharmaceuticals.
For instance, sulfuric acid is used in processes as a raw material and a reagent. As a result, it finds application in different industries, such as the automotive, pharmaceutical, steel production, and textile industries.
Interesting Science Videos
Definition Of Acids
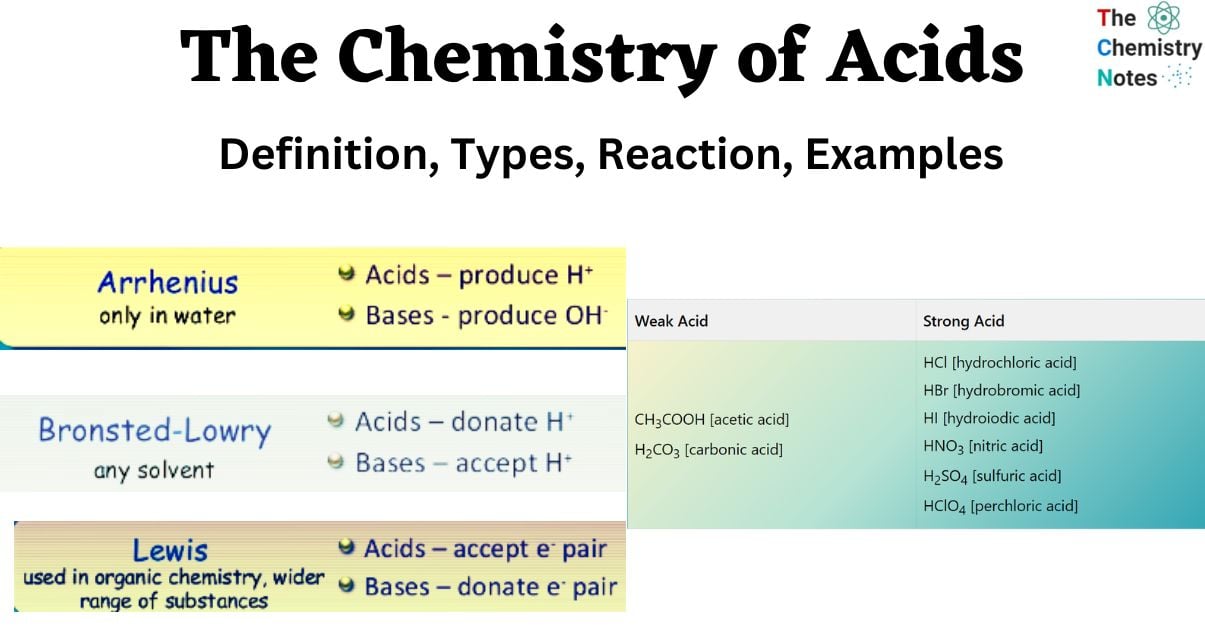
Arrhenius Definition
This definition is based on Svante Arrhenius’ proposal that when the hydrogen ion concentration in water increases, the acid dissolves more thoroughly. Therefore, an Arrhenius acid is any compound that, when dissolved in water, increases the number of hydrogen ions (H+).
In addition, Arrhenius acid is a chemical that, when dissolved in water, generates several hydrogen ions, including hydronium ions (H3O+) and other forms such as H5O2+ and H9O4+.
Bronsted–Lowry Definition
According to this theory, a base must exist for an acid to function as an acid. On the other side, an acid must be present for a base to work correctly. This thesis states that an acid is anything that donates a proton to a base, which serves as the proton acceptor. The Arrhenius theory cannot fully explain many organic processes that do not involve hydronium ions.
One example is the dynamic equilibrium reactions between acetic acid and ammonia, depicted in the following example.
CH3COOH + NH3 ⇌ CH3COO– + NH4+
Lewis Definition
In 1923, Gilbert N. Lewis put forth a theory that was an advancement on earlier hypotheses concerning acids and bases. The Lewis definition does not account for proton transfers or involve hydrogen ions in any way. In its place, it defines an acid as a material that readily takes a pair of electrons from another chemical in a solution. This is the characteristic that distinguishes an acid from other substances.
Different Types Of Acids And Their Applications
Acids are ubiquitous in our everyday lives and can be found in a wide variety of places, ranging from the physiological or biological processes that occur in our bodies to the foods and drinks that we consume.
Due to the presence of hydrochloric acid, which is one of the primary components in the digestive fluids and helps to break down food, our stomach has an acidic environment. This helps facilitate the digestion process. Similarly, the liver in our bodies creates the bile acids that are necessary for the digestion of lipids in the diet.
In addition, we consume orange juice and employ lemon juice in cooking, both of which are sources of citric acid. The food that we eat is seasoned with acetic acid. Acids are used in the manufacturing process of the plastic goods that we use. All of these things are examples of various kinds of acids that are encountered in regular life.
The following list provides some examples of common acids and their uses.
Citric acid
Citric acid is a form of organic acid that can be found naturally. Citrus fruits like lemons and oranges are a common source of natural citric acid. It has a role in the food industry as both a flavor and a preservative.
Ascorbic acid
This is another type of organic acid that occurs naturally and is usually found in citric fruits. It is a powerful form of antioxidant that is also utilized in the treatment of scurvy and disorders that affect the bone marrow. Another name for this substance is vitamin C.
Acetic acid
Acetic acid is a type of organic acid that is frequently utilized in the seasoning of food. Additionally, it is employed as a food preservative, for example, in the process of pickling.
Sulphuric acid
Sulfuric acid is a type of inorganic acid that can be used for several different purposes. It plays a role in industrial processes, such as fertilizer production, and is employed as an electrolyte in lead batteries.
Boric acid
Boric acid is also utilized in a variety of industrial processes, including the production of glass, paper, and leather, to name a few of its many uses.
Comparison Between Weak And Strong Acids
- The concentration of the acid solution is the primary factor that determines how strong or weak the acid is in terms of the pH level. When the concentration is increased, the acid’s strength also increases.
- Other factors, such as the solution’s temperature, affect more than just the pH level and acid strength. Since pH is not an accurate measurement of the concentration of an acid.
- Examining the dissociation constant, which is the ratio between the ions and the amount of acid in a solution, is one way to get a more objective measurement of the strength of an acid. When placed in an aqueous solution, stronger acids dissociate more than their weaker counterparts. This formula can demonstrate how to represent the constant.
Examples Of Weak And Types Of Acids Strong Acids
| Weak Acid | Strong Acid |
| CH3COOH [acetic acid] H2CO3 [carbonic acid] | HCl [hydrochloric acid] HBr [hydrobromic acid] HI [hydroiodic acid] HNO3 [nitric acid] H2SO4 [sulfuric acid] HClO4 [perchloric acid] |
Properties of Acid
- Acids dissolved in water form aqueous solutions that are classified as electrolytes due to their ability to conduct electricity. Certain acids are considered strong electrolytes due to their ability to fully ionize in water, resulting in a significant amount of ions. Some acids are considered weak electrolytes because they mainly exist in a non-ionized state when they are dissolved in water.
- Acids are characterized by their sour taste. Acids are present in lemons, vinegar, and sour candies.
- Certain acid-base indicators undergo a change in color when exposed to acids. Litmus and phenolphthalein are two commonly used indicators. In the presence of an acid, blue litmus changes its color to red, whereas phenolphthalein loses its color and becomes colorless.
- When active metals come into contact with acids, they undergo a chemical reaction that produces hydrogen gas.
- When acids and bases are combined, they undergo a chemical reaction that results in the formation of a salt compound and water. When an equal number of moles of an acid and a base are mixed, the acid is neutralized by the base. The reaction yields two products: an ionic compound, commonly referred to as a salt, and water.
Reactions with acids
Reaction of Acid and metal
When acid is combined with metal, the resulting reaction produces salt and hydrogen gas.
- Acid + metal → salt + hydrogen
hydrochloric acid + magnesium → magnesium chloride + hydrogen
2HCl(aq) + Mg(s) → MgCl2(aq) + H2(g)
Observations: grey solid magnesium disappears, colourless solution produced, heat released, bubbles.
The hydrogen in these reactions can be tested. We can identify gases by various reactions.
Read Also: Identification of Gases
Reaction of Acid with bases
Acids react with bases to form a salt and water.
- acid + base → salt + water
sulfuric acid + copper(II) oxide → copper(II) sulfate + water
H2SO4 (aq) + CuO (s) → CuSO4 (aq) + H2O (l)
Observations: black solid copper(II) oxide disappears and blue solution is produced.
Acid reactions with carbonates and hydrogen carbonates
Acids react with metal carbonates and hydrogen carbonates in the same way. These reactions produce salt, water and carbon dioxide.
- acid + carbonate → salt + water + carbon dioxide
hydrochloric acid + copper(II) carbonate → copper(II) chloride + water + carbon dioxide
2HCl (aq) + CuCO3 (s) → CuCl2 (aq) + H2O (l) + CO2 (g)
Observations: green solid copper(II) carbonate disappears, blue solution produced, heat released, bubbles.
- acid + hydrogen carbonate → salt + water + carbon dioxide
hydrochloric acid + sodium hydrogen carbonate → sodium chloride + water + carbon dioxide
HCl(aq) + NaHCO3 (s) → NaCl(aq) + H2O(l) + CO2(g)
Observations: solid white sodium hydrogencarbonate disappears, colourless solution produced, bubbles.
Reaction of acid with ammonia
Acids react with ammonia to form a salt.
- acid + ammonia → ammonium salt
Example:
sulfuric acid + ammonia → ammonium sulfate
H2SO4 (aq) + 2NH3 (g) → (NH4)2SO4 (aq)
References
- https://www.bbc.co.uk/bitesize/guides/
- https://edu.rsc.org/lesson-plans/reactions-of-acids-with-metals-and-carbonates-11-14-years/64.article
- https://www.bbc.co.uk/bitesize/guides/zmjyqp3/revision/7
- https://byjus.com/chemistry/acids-and-bases/
- https://www.coursehero.com/study-guides/cheminter/properties-of-acids-and-bases/
- https://www.chemicals.co.uk/blog/what-is-an-acid-in-chemistry
