The concept of thermodynamic favorability is employed to forecast the spontaneity of a chemical reaction or any other process. As previously mentioned in this unit, a spontaneous process is characterized by its ability to occur without the need for external inputs, while a nonspontaneous reaction necessitates such inputs to take place.
Thermodynamically favorable reactions are those that occur spontaneously, while thermodynamically unfavorable reactions are those that do not occur spontaneously.
We can infer whether a reaction is product- or reactant-favored by assessing the spontaneity of the reaction.
Interesting Science Videos
Thermodynamic favorability
Thermodynamic favorability refers to the intrinsic propensity of a reaction to occur spontaneously within a closed system, without requiring any external intervention.
An instance of a thermodynamically favored process is the dissolution of NaCl in water.
A process that can proceed spontaneously once the activation energy has been attained is deemed to possess thermodynamic favorability.
A process that is thermodynamically favored exhibits a directional preference, being thermodynamically favorable in one direction while being non-thermodynamically favorable in the opposite direction. However, what is the significance or interpretation of this? Water will be utilized as an illustrative instance. At a temperature of -10°C, water undergoes a phase transition from a liquid state to a solid state, however, it does not undergo the reverse transition from a solid state to a liquid state at this temperature. So,
- H2O (l) → H2O (s): is thermodynamically favored at -10 °C.
- H2O (s) → H2O(l): is non-thermodynamically favored at -10 °C.
A process that is not thermodynamically favored is one that requires external assistance to occur within the system. The aforementioned reactions can be induced to progress through the application of an extrinsic energy source.
Gibbs Free Energy and Thermodynamic Favorability
Gibbs Free Energy is a thermodynamic quantity that is utilized to ascertain the spontaneity of a chemical reaction. The Gibbs Free Energy equation is expressed as a function of temperature, ΔH°, and ΔS°.
ΔG° = ΔH° – TΔS°
Gibbs Free Energy is a thermodynamic quantity that quantifies the amount of energy that is available within a given system to perform useful work. Thus, the computation of ΔG° enables us to determine the quantity of energy that is either liberated (in the case of a negative value) or consumed (in the case of a positive value) during a reaction. It can be inferred from this point whether a reaction is exergonic, which involves the release of energy, or endergonic, which involves the absorption of energy, and consequently, whether it is spontaneous or nonspontaneous.
The equation representing the change in Gibbs free energy under standard conditions, ΔG°, can be regarded as a fusion of the two metrics that quantify the degree of spontaneity. Enthalpy and entropy are jointly accounted for in a single equation, indicating that both contribute to the thermodynamic feasibility of a process.
- When the value of ΔG° is negative, it indicates that the reaction is exergonic in nature. This implies that the reaction is spontaneous and thermodynamically favorable.
- If ΔG° is positive, the reaction is endergonic and therefore non-spontaneous and thermodynamically unfavorable.
Thermodynamic Favorability in terms of Change in Entropy and Enthalpy
Thermodynamically stated, exothermic reactions are typically favored due to their propensity to release energy to the surrounding environment. Nevertheless, certain endothermic reactions may also possess thermodynamic favorability. As an illustration, the process of evaporation is characterized by an endothermic nature and is thermodynamically favored.
The enthalpy change (ΔH) for an endothermic reaction is always positive.
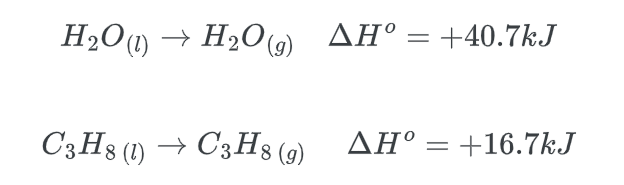
Thermodynamically favorable processes are characterized by an increase in entropy (S). According to the general principle, a thermodynamically favored reaction occurs when the entropy of the universe, including the thermodynamic system and its surroundings, is positive. If the change in entropy of the universe, represented by ΔSuniverse, is negative, then the thermodynamic favorability of the reaction is unfavorable.

It is possible to achieve a thermodynamically favorable process by combining thermodynamically unfavored reactions with thermodynamically favored reactions. The phenomenon is referred to is commonly referred to as coupling, For further ideas look into “Coupled Reactions“.
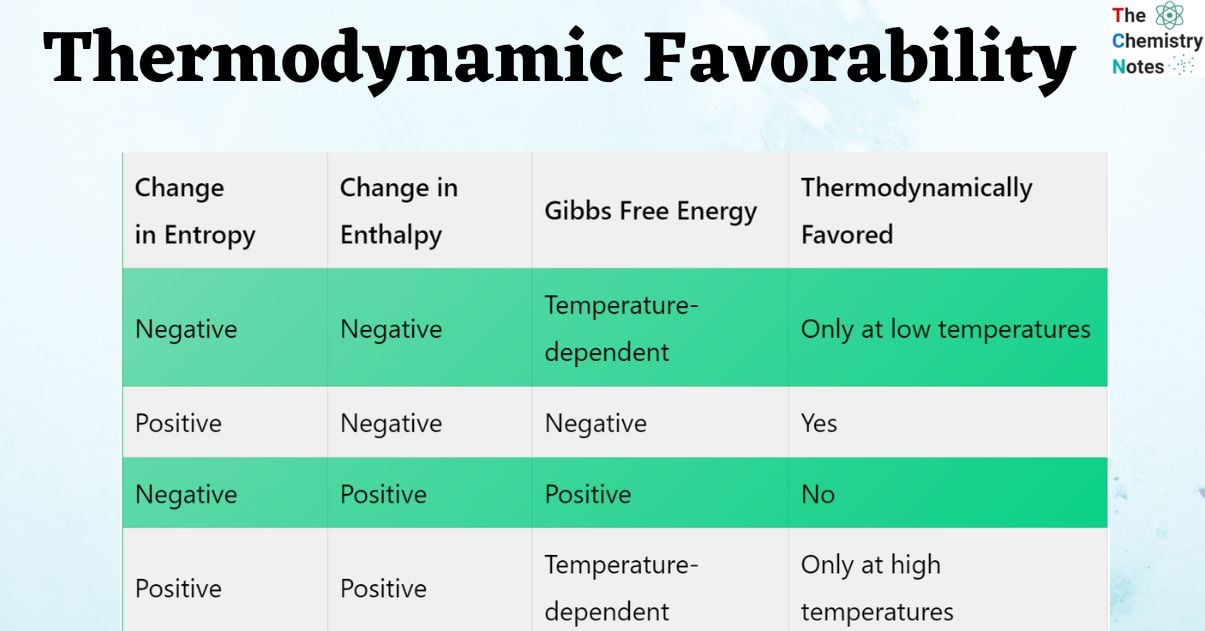
Thermodynamically favored chart
| Change in Entropy | Change in Enthalpy | Gibbs Free Energy | Thermodynamically Favored |
| Negative | Negative | Temperature-dependent | Only at low temperatures |
| Positive | Negative | Negative | Yes |
| Negative | Positive | Positive | No |
| Positive | Positive | Temperature-dependent | Only at high temperatures |
References
- Mcmahon, P. E., Rosemary Fischer Mcmahon, & Khomtchouk, B. B. (2019). Survival guide to general chemistry. Crc Press.
- https://library.fiveable.me/ap-chem/unit-9/gibbs-energy-favorability/study-guide/hCJVI2XJaSGmj1c3zvrO
- Nedu Llc. (2021). Chemistry made easy : an illustrated study guide for students to easily learn chemistry. Nurseedu.com.
- https://www.jove.com/science-education/11694/gibbs-free-energy-and-thermodynamic-favorability
- Solomons, G., Fryhle, C. B., & Snyder, S. A. (2016). Organic chemistry II. John Wiley & Sons, Inc.
- https://www.studysmarter.co.uk/explanations/chemistry/physical-chemistry/thermodynamically-favored/
