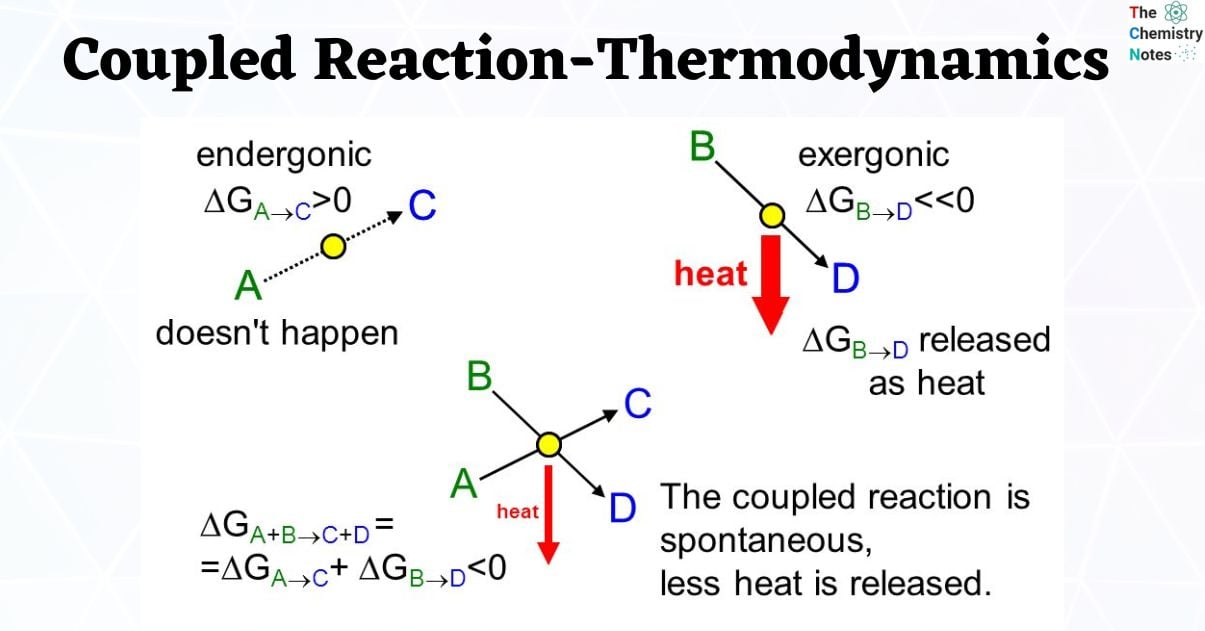
At constant temperature and pressure, G must be less than 0 for a reaction to be thermodynamically favorable. However, it is possible to force a reaction with G > 0 to proceed by adding outside energy or “coupling” an unfavorable reaction with a process that is thermodynamically favorable.
Interesting Science Videos
What are Coupled Reaction?
Many chemical processes are endergonic, or not spontaneous ( ΔG>0), and demand external energy to proceed. These reactions can, however, be related to other, exergonic (thermodynamically favorable) processes that ‘drive’ the thermodynamically unfavorable one by coupling or mechanistically connecting’ the two reactions, which frequently happen via a common intermediate.
Since Gibbs Energy is a state function, the combined Gibbs Energy of the coupled reaction can be obtained by adding the values for each half-reaction.
Example of Coupled Reaction
Decomposition of calcium carbonate is an example of coupled reaction:
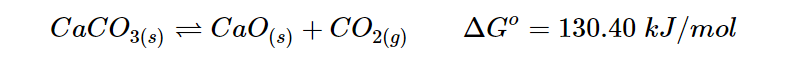
The reaction has a strongly positive ΔG, indicating that it is favored towards the reactants. When the temperature exceeds 837 ºC, the reaction becomes spontaneous and the products are favored. Now, let’s consider a second reaction that can be coupled with this reaction, which is completely different. The energy released by burning coal, which is ΔGo=−394.36 kJ/mol, is higher than the energy needed to decompose calcium carbonate, which is ΔGo=130.40 kJ/mol.
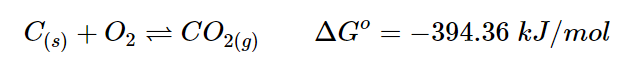
Adding two reaction:
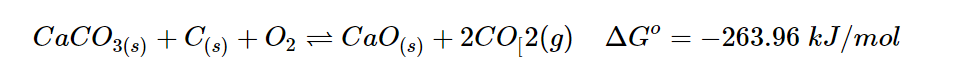
When Hess’s Law is applied, it can be observed that the combined reaction is product-favored, with a ΔGo value of -263.96 kJ/mol. The reason for this is that the reactant-favored reaction is associated with a highly spontaneous reaction, resulting in the production of products in both reactions.
It is important to note that the ΔG value for the coupled reaction is the sum of the individual reactions. This is due to the fact that Gibbs energy is a state function.
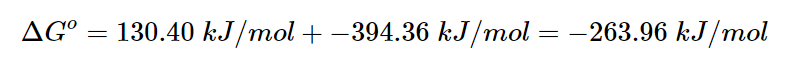
Example of Coupled Reaction in Biology
In biological systems, coupling of reactions is a common phenomenon, and some enzyme-catalyzed reactions can be understood as two coupled half-reactions, one of which is spontaneous and the other non-spontaneous.ADP (adenosine diphosphate) is produced spontaneously by the hydrolysis of ATP (adenosine triphosphate) by organisms.
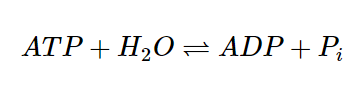
- Pi is inorganic phosphate ion
It is common to refer to the phosphoanhydride bonds generated by ejecting water between two phosphate groups of ATP as “high energy” bonds since they have a significant negative −ΔG of hydrolysis. However, like with all bonds, breaking these bonds needs energy. However, when accounting for the solvation thermodynamics of the phosphate ions, the thermodynamic Gibbs energy difference is significant “energy releasing”; the ΔG for this reaction is -31 kJ/mol.
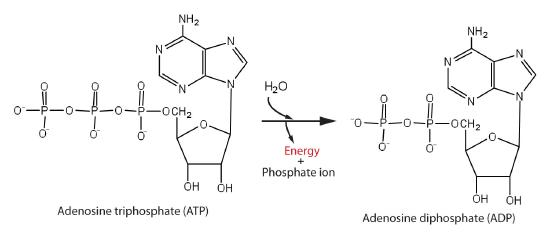
[Image source: Chem.libre]
The major ‘energy’ molecule created by metabolism is ATP, which acts as a kind of ‘energy source’ in the cell. ATP is transported to the site of any non-spontaneous processes so that the two events are coupled and the total reaction is thermodynamically favorable.
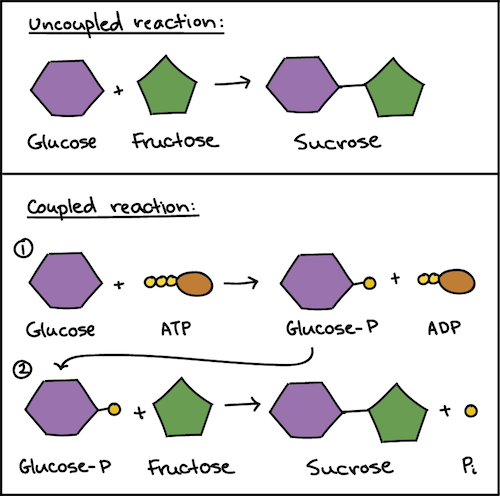
Non-spontaneous process Changing to Spontaneous through electricity
Reactions that are thermodynamically unfavorable cannot occur spontaneously and require an external energy source to take place.
Nonspontaneous processes can be facilitated by external sources of energy. Electricity is a widely used source of energy to drive nonspontaneous processes. Nonspontaneous redox reactions can occur through the use of electrical energy. An electrolytic cell can be connected to a battery to facilitate the transfer of electrons from a negatively charged ion to a positively charged ion in a reaction of the form Y+ + Z– → Y + Z (assuming Y + Z → Y+ + Z– occurs spontaneously). These cells exhibit similarities to the charging process of a battery.
Calculating ΔG° using Coupling of reaction
Calculate the ΔG° value for the reaction Fe2O3 + 3CO → 2Fe + 3CO2
- Fe2O3 → 2Fe + 3/2 O2 (ΔG° = 742.2 kJ)
- CO + 1/2 O2 → CO2 (ΔG° = -283.5 kJ)
We can use coupled reaction to solve this problem because the two reactions have a common intermediate of O2. However, we first want to multiply the bottom reaction by three to make the O2s cancel out. When we do so, we also multiply ΔG° by the same factor.
Fe2O3 → 2Fe + 3/2 O2 (ΔG° = 742.2 kJ)
3CO + 3/2 O2 → 3 CO2 (ΔG° = -283.5 * 3 kJ = -850.5 kJ)
Fe2O3 + 3CO + 3/2O2 → 2Fe + 3CO2 + 3/2O2 (ΔG° = 742.2 kJ + -850.5 kJ = -108.3 kJ)
Fe2O3 + 3CO+ → 2Fe + 3CO2 (ΔG° = -108.3 kJ)
References
- https://www.life.illinois.edu/crofts/bioph354/coupled.html
- https://chem.libretexts.org/Bookshelves/General_Chemistry/Map%3A_General_Chemistry_(Petrucci_et_al.)/19%3A_Spontaneous_Change%3A_Entropy_and_Gibbs_Energy/19.8%3A_Coupled_Reactions
- https://www2.chem.wisc.edu/deptfiles/genchem/netorial/modules/thermodynamics/free/free06.htm
- https://unacademy.com/content/csir-ugc/study-material/life-sciences/coupled-reaction-in-biology/
- https://library.fiveable.me/ap-chem/unit-9/coupled-reactions/study-guide/iNXDbmYm10NlojcZ3cu4

great work , thanks a lot.