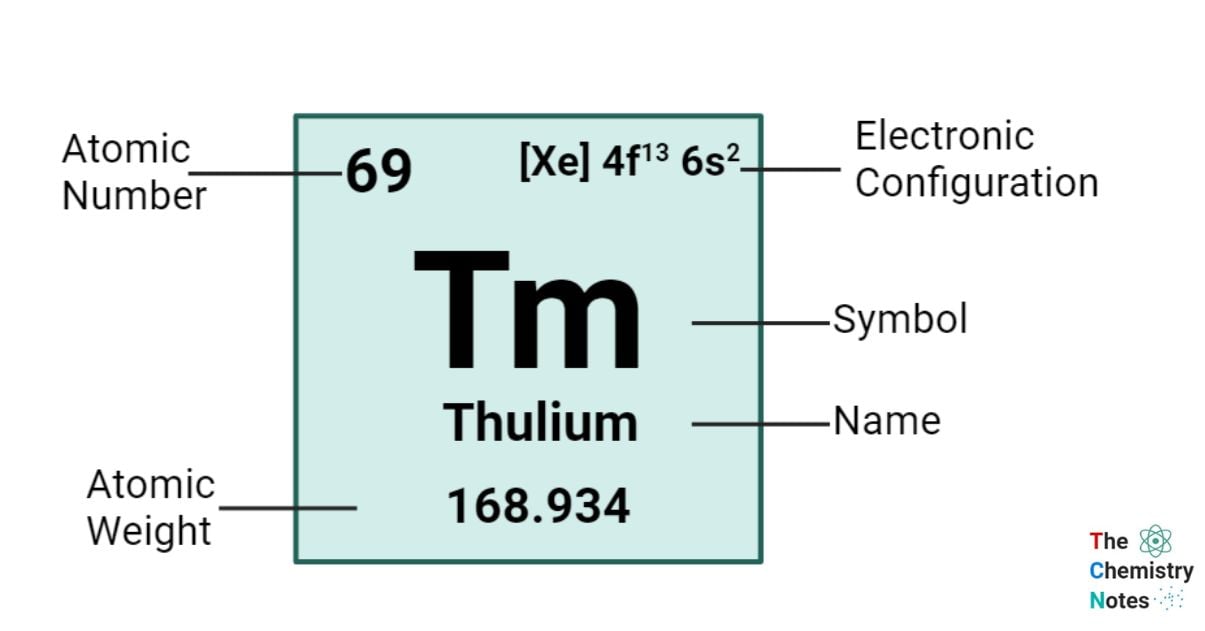
Thulium is a chemical element with an atomic number of 69 and is represented by the symbol ‘Tm’ in the periodic table. It is hard and silvery in appearance and classified as rare earth metal and belongs to the f-block of the lanthanide group of the periodic table. Thulium, like the other elements within the Lanthanides series, this particular element predominantly exhibits an oxidation state of +3. This oxidation state is evident in its diverse array of compounds, including oxides and halides. However, it is important to acknowledge that the +2 oxidation state can also demonstrate stability.
Tm is not naturally occurring in its elemental form, but rather exists within various mineral sources, predominantly including monazite, gadolinite, xenotime, and euxenite.
Interesting Science Videos
History of Thulium
- The discovery of lanthanoids, also known as rare earth elements, can be traced back to the year 1794 when Johan Gadolin made a significant breakthrough by identifying yttrium.
- Subsequently, the chemists conducted an analysis of the yttrium composition and made observations indicating the presence of contaminants comprising chemically analogous elements.
- Consequently, the isolation of two novel elements, namely terbium, and erbium, occurred in the year 1843.
- The investigation of erbium was initiated by Per Teodor Cleve in 1874, who subsequently accomplished the isolation of thulium in its oxide state in 1879 at Uppsala University in Sweden.
- In the year 1911, Theodore William Richards, an esteemed American chemist, successfully ascertained the precise atomic weight of thulium. This accomplishment was achieved through an extensive series of 15,000 thulium bromate recrystallizations, which ultimately yielded the purest form of thulium.
- The nomenclature of the element is derived from the term ‘Thule’, which denotes an archaic appellation for the region of Scandinavia.
Occurrence of Thulium
- Thulium does not manifest in its pure form in nature; rather, it is found in diverse mineral sources, primarily encompassing monazite, gadolinite, xenotime, and euxenite.
- From a commercial standpoint, the isolation process of this substance involves the utilization of ion exchange and solvent extraction techniques. The isolation of the metal can be achieved through two distinct methods: reduction of the anhydrous fluoride utilizing calcium metal, or reduction of the oxide employing lanthanum metal.
- The Earth’s crust exhibits a notable presence of this particular element, with a weight-based abundance of 0.5 milligrams per kilogram and a molar abundance of 50 parts per billion.
- Tm, an element found in soil, is present at an approximate concentration of 0.5 parts per million. However, it is important to note that this value may vary within a range of 0.4 to 0.8 parts per million.
- Tm constitutes a minute fraction of seawater, specifically 250 parts per quadrillion.
- The occurrence of Tm ore is predominantly observed in the geographical region of China. In addition to the aforementioned countries, it is worth noting that Australia, Brazil, Greenland, India, Tanzania, and the United States possess substantial reserves of thulium.
- Tm exhibits a diverse range of isotopes, totaling 32 in number, each possessing a distinct half-life. These isotopes span across the mass numbers 146 to 177, thereby contributing to the comprehensive understanding of thulium’s nuclear properties.
Isotope of Thulium
Thulium consists of only one stable isotope: 169Tm.
| Isotope | Natural Abundance (% atoms) |
|---|---|
| 169Tm | 100 |
Elemental Properties of Thulium
| Electronic Configuration | [Xe] 4f13 6s2 |
| Atomic Number | 69 |
| Atomic Weight | 168.9342 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | Lanthanides, 6, f-block |
| Density | 9.33 g/cm3 at 20 °C |
| Appearance | silvery-white |
| Van der Waals radius | unknown |
| Electron shells | 2, 8, 18, 31, 8, 2 |
| Electrons | 69 |
| Protons | 69 |
| Neutrons in the most abundant isotope | 100 |
Physical Properties of Thulium
- Thulium has an atomic number of 69 and is a silvery-white rare earth metal. It has a melting point of 1545 °C (2813 °F) and a Boiling Point of 1950 °C (3542 °F).
- Tm has a solid phase density of 9.32 g/cm3 and a liquid or molten phase density of 8.56 g/cm3.
- It is malleable which means it can be easily beaten into thin sheets without any cleavage.
- It is ductile which means it is possible to draw thin wires from it without breaking.
- Tm exhibits ferromagnetic properties when its temperature is below 32K (-241.15°C). It exhibits antiferromagnetic properties within the range of 32-56K (-241.15°C to -217.15°C). Once the temperature exceeds 56K (-217.15°C), the substance undergoes a transition and exhibits paramagnetic behavior.
- Tm, a chemical element with the atomic number 69, exhibits two prominent allotropes known as alpha thulium and beta thulium. The crystalline structure of thulium can be classified into two distinct forms: alpha thulium, which exhibits a tetragonal shape, and beta thulium, which assumes a hexagonal shape.
| Color/physical appearance | metallic, silvery-white |
| Melting point/freezing point | 1818 K (1545 °C, 2813 °F) |
| Boiling point | 2223 K (1950 °C, 3542 °F) |
| Density | 9.32 g cm-3 at 20° |
| Malleability | Yes |
| Ductility | Yes |
| Electronegativity | 1.25 (Pauling Scale) |
Chemical Properties of Thulium
- The element Tm exhibits a gradual process of tarnishing upon exposure to atmospheric conditions.
- The trivalent Tm ions (Tm3+) exhibit a pronounced emission of vibrant blue luminescence upon excitation.
- The chemical element Tm exhibits reactivity when exposed to water, resulting in the formation of thulium hydroxide. The reaction of the substance exhibits a sluggish response when exposed to cold water while displaying an accelerated rate of reaction when subjected to hot water.
- Tm exhibits reactivity with various halogens, resulting in the formation of the respective trihalides. The reaction exhibits a sluggish rate at ambient conditions while displaying significantly enhanced reactivity when subjected to elevated temperatures of approximately 200°C.
- Tm predominantly adopts the trivalent oxidation state, represented as Tm3+, in its chemical compound configurations. The chemical element possesses the capacity to undergo chemical reactions with both oxygen and halogens, leading to the creation of compounds that are primarily distinguished by their prominent green hue.
- Tm exhibits a pronounced propensity to undergo combustion in the presence of atmospheric oxygen at a temperature of 150°C, resulting in the formation of thulium(III) oxide.
Chemical Reaction of Thulium
- The Reaction of Thulium With Air
Tm metal undergoes gradual oxidation in the presence of atmospheric oxygen, resulting in the formation of Tm (III) oxide, denoted as Tm2O3, which exhibits a propensity for combustion.
4 Tm (s) + 3 O2 (g) → 2 Tm2O3 (s)- The Reaction of Thulium With Water
Tm exhibits a sluggish reaction rate when exposed to cold water, whereas it undergoes a rapid reaction when in contact with hot water. This reaction results in the formation of Tm (III) hydroxide, denoted as Tm(OH)3, along with the liberation of hydrogen gas, H2.
2 Tm (s) + 6 H2O (l) → 2 Tm(OH)3 (aq) + 3 H2 (g)- The Reaction of Thulium With Halogens
The element terbium exhibits a propensity to engage in chemical reactions with various halogens, resulting in the formation of Tm (III) halides.
The chemical reaction between Tm metal and fluorine gas (F2) results in the formation of thulium (III) fluoride, denoted as TmF3.
2 Tm (s) + 3 F2 (g) → 2 TmF3 (s) [White]The chemical reaction between Tm metal and chlorine gas (Cl2) results in the formation of thulium (III) chloride, denoted as TmCl3.
2 Tm (s) + 3 Cl2 (g) → 2 TmCl3 (s) [Yellow]The chemical reaction between Tm metal and bromine (Br2) results in the formation of thulium (III) bromide, denoted as TmBr3.
2 Tm (s) + 3 Br2 (g) → 2 TmBr3 (s) [White]The chemical reaction between Tm metal and iodine represented as (I2), results in the formation of thulium (III) iodide, denoted as TmI3.
2 Tm (s) + 3 I2 (g) → 2 TmI3 (s) [Yellow]- The Reaction of Thulium With Acid
Tm metal has a high degree of solubility in diluted sulfuric acid, which results in the formation of solutions containing the light-green aquatic Tm(III) ion and the release of Hydrogen gas, H2. There is a high probability that Tm3+(aq) predominantly exists in the form of the complex ion [Tm(OH2)9]3+.
2 Tm (s) + 3 H2SO4 (aq) → 2 Tm3+ (aq) + 3 SO42- (aq) + 3 H2 (g)Uses of Thulium
The metal has very few commercial applications because of its high cost and low relative abundance. Some applications exist for Tm and its compounds some of which are discussed here:
Used In Lasers
The substance referred to as holmium-chromium-thulium triple-doped yttrium aluminum garnet, commonly denoted as (Ho:Cr:Tm: YAG) or (Ho:Cr:Tm: YAG) is a noteworthy active laser medium material renowned for its exceptional efficiency. The laser emits radiation with a wavelength of 2080 nm, falling within the infrared spectrum. This particular laser has found significant utility in diverse domains, including military operations, medical practices, and meteorological studies.
Used In X-rays
Despite the considerable expense associated with portable X-ray devices, they employ Tm which has undergone neutron bombardment within a nuclear reactor to generate the Thulium-170 isotope. This particular isotope possesses a half-life of 128.6 days and exhibits five primary emission lines of similar intensity. The radioactive sources possess a practical lifespan of approximately one year and are employed as instruments in the field of medical and dental diagnostics, as well as for the purpose of identifying flaws in mechanical and electronic components that are not easily accessible.
Miscellaneous Uses
- High-temperature superconductors are known to incorporate this particular material.
- Ceramic magnetic materials have been widely employed in the domain of microwaves.
- Electricity-generating windows, operating on the fundamental principle of a luminescent solar concentrator, have the potential for utilization.
Effects on Health
- Tm exhibits a relatively low level of toxicity; however, it is important to note that at elevated concentrations, it can pose potential hazards. It is noteworthy that thulium salts, despite their insolubility, exhibit the remarkable characteristic of being entirely devoid of toxicity.
- Ingestion of this substance has been found to elicit deleterious effects on the liver and spleen. The inhalation or ingestion of thulium dust poses significant risks and can have detrimental effects on human health. The phenomenon under consideration possesses the potential to induce explosive reactions.
Effects on Environment
- Tm does not present any discernible deleterious effects on the well-being of plants, animals, or the environment.
References
- https://www.rsc.org/periodic-table/element/69/thulium
- https://www.chemicool.com/elements/thulium.html’
- www.sciehttps://www.lenntech.com/periodic/elements/tm.htm#:~:text=Thulium%20is%20a%20lanthanide%20element,than%20most%20rare%2Dearth%20elements.ncedirect.com/topics/chemical-engineering/tm
- Robert E. Krebs, The history and use of our earth’s chemical elements: a reference guide., JGreenwood Publishing Group, 2006, p300.
- https://www.vedantu.com/chemistry/thulium
- https://education.jlab.org/itselemental/ele069.html

